CLDN6-specific CAR-T cells plus amplifying RNA vaccine in relapsed or refractory solid tumors: the phase 1 BNT211-01 trial
- PMID: 37872225
- PMCID: PMC10667102
- DOI: 10.1038/s41591-023-02612-0
CLDN6-specific CAR-T cells plus amplifying RNA vaccine in relapsed or refractory solid tumors: the phase 1 BNT211-01 trial
Abstract
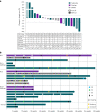
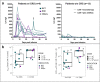
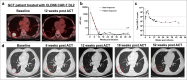
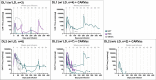
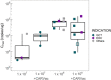
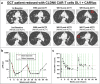
The oncofetal antigen Claudin 6 (CLDN6) is highly and specifically expressed in many solid tumors, and could be a promising treatment target. We report dose escalation results from the ongoing phase 1/2 BNT211-01 trial evaluating the safety and feasibility of chimeric antigen receptor (CAR) T cells targeting the CLDN6 with or without a CAR-T cell-amplifying RNA vaccine (CARVac) at two dose levels (DLs) in relapsed/refractory CLDN6-positive solid tumors. The primary endpoints were safety and tolerability, maximum tolerated dose and recommended phase 2 dose (RP2D). Secondary endpoints included objective response rate (ORR) and disease control rate. We observed manageable toxicity, with 10 out of 22 patients (46%) experiencing cytokine release syndrome including one grade 3 event and 1 out of 22 (5%) with grade 1 immune effector cell-associated neurotoxicity syndrome. Dose-limiting toxicities occurred in two patients at the higher DL, resolving without sequelae. CAR-T cell engraftment was robust, and the addition of CARVac was well tolerated. The unconfirmed ORR in 21 evaluable patients was 33% (7 of 21), including one complete response. The disease control rate was 67% (14 of 21), with stable disease in seven patients. Patients with germ cell tumors treated at the higher DL exhibited the highest response rate (ORR 57% (4 of 7)). The maximum tolerated dose and RP2D were not established as the trial has been amended to utilize an automated manufacturing process. A repeat of the dose escalation is ongoing and will identify a RP2D for pivotal trials. ClinicalTrials.gov Identifier: NCT04503278 .
© 2023. The Author(s).
Conflict of interest statement
Y.H., T.H., Q.K.-F., A.M.S., C.S.-E., A.F., C.F., K.K., L.P., B.R., Ö.T. and U.Ş. are employees at BioNTech SE. E.W. previously worked as consultants to BioNTech SE. U.Ş. and Ö.T. are management board members of BioNTech SE (Mainz, Germany). U.Ş., Ö.T. and B.R. are inventors on patents and patent applications, which cover parts of this article. The remaining authors declare nocompeting interests.
Figures




Comment in
-
Boosting CAR T cells with anti-tumor mRNA vaccines.Nat Med. 2023 Nov;29(11):2711-2712. doi: 10.1038/s41591-023-02623-x. Nat Med. 2023. PMID: 37932550 No abstract available.
References
-
- Berdeja JG, et al. Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE-1): a phase 1b/2 open-label study. Lancet. 2021;398:314–324. doi: 10.1016/S0140-6736(21)00933-8. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

