A novel mouse model of mitochondrial disease exhibits juvenile-onset severe neurological impairment due to parvalbumin cell mitochondrial dysfunction
- PMID: 37872380
- PMCID: PMC10593770
- DOI: 10.1038/s42003-023-05238-7
A novel mouse model of mitochondrial disease exhibits juvenile-onset severe neurological impairment due to parvalbumin cell mitochondrial dysfunction
Abstract
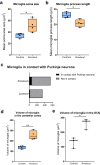
Mitochondrial diseases comprise a common group of neurometabolic disorders resulting from OXPHOS defects, that may manifest with neurological impairments, for which there are currently no disease-modifying therapies. Previous studies suggest inhibitory interneuron susceptibility to mitochondrial impairment, especially of parvalbumin-expressing interneurons (PV+). We have developed a mouse model of mitochondrial dysfunction specifically in PV+ cells via conditional Tfam knockout, that exhibited a juvenile-onset progressive phenotype characterised by cognitive deficits, anxiety-like behaviour, head-nodding, stargazing, ataxia, and reduced lifespan. A brain region-dependent decrease of OXPHOS complexes I and IV in PV+ neurons was detected, with Purkinje neurons being most affected. We validated these findings in a neuropathological study of patients with pathogenic mtDNA and POLG variants showing PV+ interneuron loss and deficiencies in complexes I and IV. This mouse model offers a drug screening platform to propel the discovery of therapeutics to treat severe neurological impairment due to mitochondrial dysfunction.
© 2023. Springer Nature Limited.
Conflict of interest statement
The authors declare no competing interests.
Figures
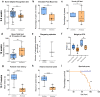
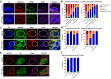
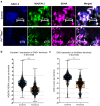
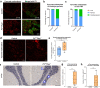




References
-
- Gorman GS, et al. Mitochondrial diseases. Nat. Rev. Dis. Primers. 2016;2:16080. - PubMed
-
- Ng YS, et al. Mitochondrial disease in adults: recent advances and future promise. Lancet Neurol. 2021;20:573–584. - PubMed
-
- Iizuka T, et al. Neuronal hyperexcitability in stroke-like episodes of MELAS syndrome. Neurology. 2002;59:816. - PubMed
-
- Tzoulis C, Bindoff LA. Acute mitochondrial encephalopathy reflects neuronal energy failure irrespective of which genome the genetic defect affects. Brain. 2012;135:3627–3634. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

