Phase II study of everolimus and temozolomide as first-line treatment in metastatic high-grade gastroenteropancreatic neuroendocrine neoplasms
- PMID: 37872405
- PMCID: PMC10703888
- DOI: 10.1038/s41416-023-02462-0
Phase II study of everolimus and temozolomide as first-line treatment in metastatic high-grade gastroenteropancreatic neuroendocrine neoplasms
Abstract
Background: The optimal treatment for metastatic high-grade gastroenteropancreatic (GEP) neuroendocrine neoplasms when Ki-67 ≤55% is unknown. A prospective multi-centre phase 2 study was performed to evaluate the efficacy and safety of everolimus and temozolomide as first-line treatment for these patients.
Methods: Patients received everolimus 10 mg daily continuously and temozolomide 150 mg/m2 for 7 days every 2 weeks. Endpoints included response, survival, safety and quality of life (QoL). Histopathological re-evaluation according to the 2019 WHO classification was performed.
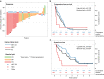
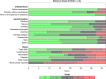
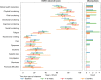
Results: For 37 eligible patients, the primary endpoint with 65% disease control rate (DCR) at 6 months (m) was reached. The response rate was 30%, the median progression-free survival (PFS) 10.2 months and the median overall survival (OS) 26.4 months. Considering 26 NET G3 patients, 6 months DCR was 77% vs. 22% among nine NEC patients (p = 0.006). PFS was superior for NET G3 vs. NEC (12.6 months vs. 3.4 months, Log-rank-test: p = 0.133, Breslow-test: p < 0.001). OS was significantly better for NET G3 (31.4 months vs. 7.8 months, p = 0.003). Grade 3 and 4 toxicities were reported in 43% and 38%. QoL remained stable during treatment.
Conclusion: Everolimus and temozolomide may be a treatment option for selected GEP-NET G3 patients including careful monitoring. Toxicity did not compromise QoL.
Clinical trial registration: ClinicalTrials.gov (NTC02248012).
© 2023. The Author(s).
Conflict of interest statement
HS: Consultant/advisory board: Hutchison, Bayer, ITM, AAA. Lecture Honoraria; Novartis, Ipsen, Bayer, SAM Nordic, Pierre Fabre. ML: Unrestricted research grant from Scandion Oncology A/S.
Figures
References
-
- Milione M, Maisonneuve P, Spada F, Pellegrinelli A, Spaggiari P, Albarello L, et al. The clinicopathologic heterogeneity of grade 3 gastroenteropancreatic neuroendocrine neoplasms: morphological differentiation and proliferation identify different prognostic categories. Neuroendocrinology. 2017;104:85–93. - PubMed
-
- Heetfeld M, Chougnet CN, Olsen IH, Rinke A, Borbath I, Crespo G, et al. Characteristics and treatment of patients with G3 gastroenteropancreatic neuroendocrine neoplasms. Endocr Relat Cancer. 2015;22:657–64. - PubMed

