Development and Validation of a New Hierarchical Composite End Point for Clinical Trials of Kidney Disease Progression
- PMID: 37872654
- PMCID: PMC10703083
- DOI: 10.1681/ASN.0000000000000243
Development and Validation of a New Hierarchical Composite End Point for Clinical Trials of Kidney Disease Progression
Abstract
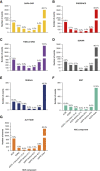
Significance statement: The established composite kidney end point in clinical trials combines clinical events with sustained large changes in GFR but does not weigh the relative clinical importance of the end point components. By contrast, a hierarchical composite end point (HCE) accounts for the clinical importance of the end point components. The authors developed and validated a kidney HCE that combines clinical kidney outcomes with longitudinal GFR changes (GFR slope). They demonstrate that in seven major placebo-controlled kidney outcome trials with different medications, treatment effect estimates on the HCE were consistently in similar directions and of similar magnitudes compared with treatment effects on the established kidney end point. The HCE's prioritization of clinical outcomes and ability to combine dichotomous outcomes with GFR slope make it an attractive alternative to the established kidney end point.
Background: The established composite kidney end point in clinical trials combines clinical events with sustained large changes in GFR. However, the statistical method does not weigh the relative clinical importance of the end point components. A HCE accounts for the clinical importance of the end point components and enables combining dichotomous outcomes with continuous measures.
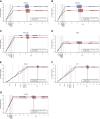
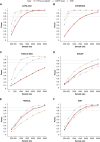
Methods: We developed and validated a new HCE for kidney disease progression, performing post hoc analyses of seven major Phase 3 placebo-controlled trials that assessed the effects of canagliflozin, dapagliflozin, finerenone, atrasentan, losartan, irbesartan, and aliskiren in patients with CKD. We calculated the win odds (WOs) for treatment effects on a kidney HCE, defined as a hierarchical composite of all-cause mortality; kidney failure; sustained 57%, 50%, and 40% GFR declines from baseline; and GFR slope. The WO describes the odds of a more favorable outcome for receiving the active compared with the control. We compared the WO with the hazard ratio (HR) of the primary kidney outcome of the original trials.
Results: In all trials, treatment effects calculated with the WO reflected a similar direction and magnitude of the treatment effect compared with the HR. Clinical trials incorporating the HCE would achieve increased statistical power compared with the established composite end point at equivalent sample sizes.
Conclusions: In seven major kidney clinical trials, the WO and HR provided similar direction of treatment effect estimates with smaller HRs associated with larger WOs. The prioritization of clinical outcomes and inclusion of broader composite end points makes the HCE an attractive alternative to the established kidney end point.
Trial registration: ClinicalTrials.gov NCT03036150 NCT02065791 NCT02540993 NCT01858532 NCT00308347 NCT00317915 NCT00549757.
Copyright © 2023 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Society of Nephrology.
Conflict of interest statement
G. Bakris is supported by T32 NIH grant DK07011 and is a consultant to Alnylam, AstraZeneca, Bayer, Glaxo Smith Kline, InREGEN, Ionis, Janssen, KBP Biosciences, and Novo Nordisk. M. Brinker reports ownership interest: Bayer AG. M. Brinker, R. Nkulikiyinka, P. Schloemer, and C. Tasto are Bayer employees. S.B. Gasparyan, M. Karpefors, D.J. Little, and J. Rossert are AstraZeneca employees. H.L. Heerspink is consultant for AstraZeneca, Bayer, Boehringer Ingelheim, Chinook, CSL Behring, Dimerix, Eli-Lilly, Gilead, Janssen, Merck, Novo Nordisk, ProKidney, Travere Therapeutics, and Vifor Fresenius. He has received research support from AstraZeneca, Boehringer Ingelheim, Janssen, and Novo Nordisk; honoraria: lecture fees from AstraZeneca and NovoNordisk; and speakers bureau: AstraZeneca. N. Jongs reports travel grants from AstraZeneca. D.J. Little reports ownership interest: AstraZeneca and other interests or relationships: volunteer as a nephrologist at Walter Reed National Military Medical Center. V. Perkovic serves as a Board Director for St. Vincents Health Australia, George Clinical, and several Medical Research Institutes. He has received honoraria for Steering Committee roles and scientific presentations and/or advisory board attendance from Abbvie, Amgen, AstraZeneca, Baxter, Bayer, Boehringer Ingelheim, Chinook, Durect, Eli Lilly, Gilead, GSK, Janssen, Merck, Mitsubishi Tanabe, Mundipharma, Novartis, Novo Nordisk, Otsuka, Pfizer, Pharmalink, Reata, Relypsa, Roche, Sanofi, Servier, Travere, and Tricida. J. Rossert reports ownership interest: Amgen, AstraZeneca, and Vertex. C. Tasto reports ownership interest: Bayer AG. D.C. Wheeler has received honoraria and/or consultancy fees from Amgen, Astellas, AstraZeneca, Boehringer Ingelheim, Bayer, Eledon, Galderma, George Clinical, Gilead, GlaxoSmithKline, Janssen, Medscape, Merck Sharp and Dohme, Mundipharma, Napp, Pfizer, Pharmacosmos, ProKidney, Reata, Takeda, Tricida, Vifor Fresenius, and Zydus and advisory or leadership role: AstraZeneca; and speakers bureau: Amgen, Astellas, AstraZeneca, Janssen, Merck Sharp and Dohme, Mundipharma, Napp, and Vifor Fresenius. R. Nkulikiyinka reports Employer: Bayer AG; and Ownership Interest: Bayer AG. G. Bakris reports Consultancy: Janssen, Bayer, KBP Biosciences, Novo Nordisk, Astra-Zeneca, Ionis, Alnylam, Medscape; Honoraria: Merck, Novo Nordisk, Astra Zeneca, Ionis, Alnylam, KBP Biosciences, and Bayer; Advisory or Leadership Role: KBP Biosciences, American J Nephrology, Editor, Diabetes Care, Assoc. Ed.,; American Heart Assoc.; UpToDate-Nephrology; and Other Interests or Relationships: American Diabetes Association, American Heart Association, Blood Pressure Council. V. Perkovic reports Consultancy: AstraZeneca, Bayer, Boehringer Ingelheim, Chinook, Eli Lilly, Gilead, GlaxoSmithKline, Janssen, Mitsubishi Tanabe, Mundipharma, Novartis, Novo Nordisk, Otsuka, Travere, Tricida, UptoDate; Ownership Interest: George Clinical; Research Funding: AstraZeneca, Bayer, Chinook, Gilead, GlaxoSmithKline, Janssen, Novartis, Novo Nordisk, Otsuka, Travere, Tricida,; Honoraria: AstraZeneca, Bayer, Boehringer Ingelheim, Chinook, Eli Lilly, Gilead, GlaxoSmithKline, Janssen, Mitsubishi Tanabe, Mundipharma, Novartis, Novo Nordisk, Otsuka, Travere, Tricida, UptoDate; and Advisory or Leadership Role: Steering committees for Bayer, Chinook, GlaxoSmithKline, Janssen, Novartis, Novo Nordisk, Otsuka, Pfizer, Travere; Board Director for St Vincents Health Australia, George Clinical.
Figures



References
-
- Levey AS Gansevoort RT Coresh J, et al. . Change in albuminuria and GFR as end points for clinical trials in early stages of CKD: a scientific workshop sponsored by the National Kidney Foundation in collaboration with the US Food and Drug Administration and European Medicines Agency. Am J Kidney Dis. 2020;75(1):84–104. doi:10.1053/j.ajkd.2019.06.009 - DOI - PubMed
-
- Lambers Heerspink HJ Weldegiorgis M Inker LA, et al. . Estimated GFR decline as a surrogate end point for kidney failure: a post hoc analysis from the reduction of end points in non–insulin-dependent diabetes with the angiotensin II antagonist losartan (RENAAL) study and irbesartan diabetic nephropathy trial (IDNT). Am J Kidney Dis. 2014;63(2):244–250. doi:10.1053/j.ajkd.2013.09.016 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

