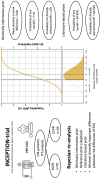
Extracorporeal life support in cardiac arrest: a post hoc Bayesian re-analysis of the INCEPTION trial
- PMID: 37872725
- PMCID: PMC10873541
- DOI: 10.1093/ehjacc/zuad130
Extracorporeal life support in cardiac arrest: a post hoc Bayesian re-analysis of the INCEPTION trial
Abstract
Aims: Previously, we performed the multicentre INCEPTION trial, randomizing patients with refractory out-of-hospital cardiac arrest (OHCA) to extracorporeal cardiopulmonary resuscitation (ECPR) or conventional cardiopulmonary resuscitation (CCPR). Frequentist analysis showed no statistically significant treatment effect for the primary outcome; 30-day survival with a favourable neurologic outcome (cerebral performance category score of 1-2). To facilitate a probabilistic interpretation of the results, we present a Bayesian re-analysis of the INCEPTION trial.
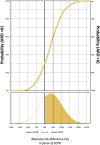
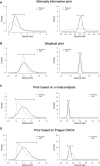
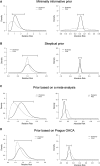
Methods and results: We analysed survival with a favourable neurologic outcome at 30 days and 6 months under a minimally informative prior in the intention-to-treat population. Effect sizes are presented as absolute risk differences (ARDs) and relative risks (RRs), with 95% credible intervals (CrIs). We estimated posterior probabilities at various thresholds, including the minimal clinically important difference (MCID) (5% ARD), based on expert consensus, and performed sensitivity analyses under sceptical and literature-based priors. The mean ARD for 30-day survival with a favourable neurologic outcome was 3.6% (95% CrI -9.5-16.7%), favouring ECPR, with a median RR of 1.22 (95% CrI 0.59-2.51). The posterior probability of an MCID was 42% at 30 days and 42% at 6 months, in favour of ECPR.
Conclusion: Bayesian re-analysis of the INCEPTION trial estimated a 42% probability of an MCID between ECPR and CCPR in refractory OHCA in terms of 30-day survival with a favourable neurologic outcome.
Trial registration: Clinicaltrials.gov (NCT03101787, registered 5 April 2017).
Keywords: Bayesian analysis; Cardiopulmonary resuscitation; Extracorporeal membrane oxygenation; Out-of-hospital cardiac arrest; Randomized controlled trial.
© The Author(s) 2023. Published by Oxford University Press on behalf of the European Society of Cardiology.
Conflict of interest statement
Conflict of interest: R.L. reports consulting fees from Abiomed and participates in an advisory board of Xenios not related to this work. All other authors report no conflicts of interest.
Figures

References
-
- Yannopoulos D, Bartos J, Raveendran G, Walser E, Connett J, Murray TA, et al. Advanced reperfusion strategies for patients with out-of-hospital cardiac arrest and refractory ventricular fibrillation (ARREST): a phase 2, single centre, open-label, randomised controlled trial. Lancet 2020;396:1807–1816. - PMC - PubMed
-
- Belohlavek J, Smalcova J, Rob D, Franek O, Smid O, Pokorna M, et al. Effect of intra-arrest transport, extracorporeal cardiopulmonary resuscitation, and immediate invasive assessment and treatment on functional neurologic outcome in refractory out-of-hospital cardiac arrest: a randomized clinical trial. JAMA 2022;327:737–747. - PMC - PubMed
-
- Suverein MM, Delnoij TSR, Lorusso R, Brandon Bravo Bruinsma GJ, Otterspoor L, Elzo Kraemer CV, et al. Early extracorporeal CPR for refractory out-of-hospital cardiac arrest. N Engl J Med 2023;388:299–309. - PubMed
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

