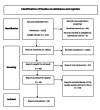
Tenofovir-Induced Renal Dysfunction Among HIV-Infected Patients: A Systematic Review
- PMID: 37872903
- PMCID: PMC10590624
- DOI: 10.7759/cureus.45787
Tenofovir-Induced Renal Dysfunction Among HIV-Infected Patients: A Systematic Review
Abstract
Tenofovir disoproxil fumarate (TDF) is an antiretroviral drug widely used as part of antiretroviral therapy (ART) to treat human immunodeficiency virus (HIV-1) infection. Negative effects of tenofovir include impaired kidney function, especially with long-term use. In studies conducted among HIV-positive individuals, we found evidence of extensive kidney damage associated with TDF use. Despite the therapeutic importance of this consequence, its continued use in ART regimens was not contraindicated. The therapeutic and long-term effects of TDF are a major concern. However, in countries or settings where resources are limited and renal function monitoring cannot be ensured, screening methods to detect ART-related renal failure are still supported by data. Therefore, it is safe to re-evaluate the use of TDF-based ART. However, adherence to guidelines may be hampered by insufficient laboratory testing in low- and middle-income countries. More research is also needed among people under 18 years of age and pregnant and breastfeeding mothers.
Keywords: glomerular and tubular injury; hiv aids; kidney failure; tenofovir alafenamide (taf); tenofovir disoproxil fumarate (tdf).
Copyright © 2023, Shivakumar et al.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Estimated glomerular filtration rate slopes on tenofovir alafenamide. Ibrahim F, Campbell L, Bailey AC, et al. HIV Med. 2020;21:607–612. - PubMed
-
- Renal toxicity of tenofovir: narrative review. Ali HH Aziz TA. Al-Rafidain J Med Sci. 2022;2:40–50.
Publication types
LinkOut - more resources
Full Text Sources