This is a preprint.
MAP1B Regulates Cortical Neuron Interstitial Axon Branching Through the Tubulin Tyrosination Cycle
- PMID: 37873083
- PMCID: PMC10592918
- DOI: 10.1101/2023.10.02.560024
MAP1B Regulates Cortical Neuron Interstitial Axon Branching Through the Tubulin Tyrosination Cycle
Update in
-
Microtubule-binding protein MAP1B regulates interstitial axon branching of cortical neurons via the tubulin tyrosination cycle.EMBO J. 2024 Apr;43(7):1214-1243. doi: 10.1038/s44318-024-00050-3. Epub 2024 Feb 22. EMBO J. 2024. PMID: 38388748 Free PMC article.
Abstract
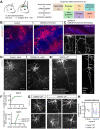
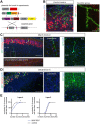
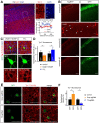
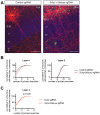
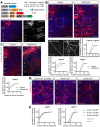
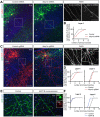
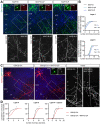
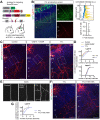
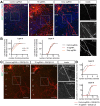
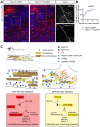
Regulation of directed axon guidance and branching during development is essential for the generation of neuronal networks. However, the molecular mechanisms that underlie interstitial axon branching in the mammalian brain remain unresolved. Here, we investigate interstitial axon branching in vivo using an approach for precise labeling of layer 2/3 callosal projection neurons (CPNs), allowing for quantitative analysis of axonal morphology at high acuity and also manipulation of gene expression in well-defined temporal windows. We find that the GSK3β serine/threonine kinase promotes interstitial axon branching in layer 2/3 CPNs by releasing MAP1B-mediated inhibition of axon branching. Further, we find that the tubulin tyrosination cycle is a key downstream component of GSK3β/MAP1B signaling. We propose that MAP1B functions as a brake on axon branching that can be released by GSK3β activation, regulating the tubulin code and thereby playing an integral role in sculpting cortical neuron axon morphology.
Keywords: Interstitial axon branching; cortical neuron development; intracellular signaling; microtubules.
Conflict of interest statement
DECLARATION OF INTERESTS The authors declare no competing interests. RH currently serves as a Director of Neuroscience at Prilenia Therapeutics. JD currently works at Novartis.
Figures

References
-
- Winnubst J., Bas E., Ferreira T.A., Wu Z., Economo M.N., Edson P., Arthur B.J., Bruns C., Rokicki K., Schauder D., et al. (2019). Reconstruction of 1,000 Projection Neurons Reveals New Cell Types and Organization of Long-Range Connectivity in the Mouse Brain. Cell 179, 268–281.e13. 10.1016/j.cell.2019.07.042. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
