This is a preprint.
G6PD Maintains Redox Homeostasis and Biosynthesis in LKB1-Deficient KRAS-Driven Lung Cancer
- PMID: 37873106
- PMCID: PMC10592603
- DOI: 10.1101/2023.10.06.561131
G6PD Maintains Redox Homeostasis and Biosynthesis in LKB1-Deficient KRAS-Driven Lung Cancer
Update in
-
Glucose-6-phosphate dehydrogenase maintains redox homeostasis and biosynthesis in LKB1-deficient KRAS-driven lung cancer.Nat Commun. 2024 Jul 12;15(1):5857. doi: 10.1038/s41467-024-50157-8. Nat Commun. 2024. PMID: 38997257 Free PMC article.
Abstract
Cancer cells depend on nicotinamide adenine dinucleotide phosphate (NADPH) to combat oxidative stress and support reductive biosynthesis. One major NAPDH production route is the oxidative pentose phosphate pathway (committed step: glucose-6-phosphate dehydrogenase, G6PD). Alternatives exist and can compensate in some tumors. Here, using genetically-engineered lung cancer model, we show that ablation of G6PD significantly suppresses KrasG12D/+;Lkb1-/- (KL) but not KrasG12D/+;p53-/- (KP) lung tumorigenesis. In vivo isotope tracing and metabolomics revealed that G6PD ablation significantly impaired NADPH generation, redox balance and de novo lipogenesis in KL but not KP lung tumors. Mechanistically, in KL tumors, G6PD ablation caused p53 activation that suppressed tumor growth. As tumor progressed, G6PD-deficient KL tumors increased an alternative NADPH source, serine-driven one carbon metabolism, rendering associated tumor-derived cell lines sensitive to serine/glycine depletion. Thus, oncogenic driver mutations determine lung cancer dependence on G6PD, whose targeting is a potential therapeutic strategy for tumors harboring KRAS and LKB1 co-mutations.
Keywords: G6PD; KRAS; LKB1; NADPH; NSCLC; Redox; fatty acid synthesis; metabolic reprogramming; serine metabolism.
Conflict of interest statement
Potential Conflicts of Interest E. W. is a stockholder in a founder of Vescor Therapeutics. J. D. R. is an advisor and stockholder in Colorado Research Partners, L.E.A.F. Pharmaceuticals, Bantam Pharmaceuticals, Rafael Pharmaceuticals; a paid consultant of Third Rock Ventures; a founder, director and stockholder of Farber Partners, Serien Therapeutics and Sofro Pharmaceuticals; a founder and stockholder in Empress Therapeutics; and a director of the Princeton University–PKU Shenzhen collaboration. The Rabinowitz lab at Princeton University and the Princeton University-PKU Shenzhen collaboration have discovered and generated intellectual property regarding G6PD inhibitors. Other authors have no conflict of interest to declare.
Figures


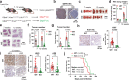
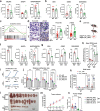


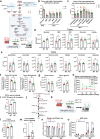
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
