This is a preprint.
Allele biased transcription factor binding across human brain regions gives mechanistic insight into eQTLs
- PMID: 37873117
- PMCID: PMC10592666
- DOI: 10.1101/2023.10.06.561245
Allele biased transcription factor binding across human brain regions gives mechanistic insight into eQTLs
Update in
-
Allele-specific transcription factor binding across human brain regions offers mechanistic insight into eQTLs.Genome Res. 2024 Sep 20;34(8):1224-1234. doi: 10.1101/gr.278601.123. Genome Res. 2024. PMID: 39152038 Free PMC article.
Abstract
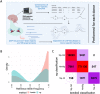
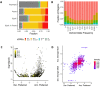
Transcription Factors (TFs) influence gene expression by facilitating or disrupting the formation of transcription initiation machinery at particular genomic loci. Because genomic localization of TFs is in part driven by TF recognition of DNA sequence, variation in TF binding sites can disrupt TF-DNA associations and affect gene regulation. To identify variants that impact TF binding in human brain tissues, we quantified allele bias for 93 TFs analyzed with ChIP-seq experiments of multiple structural brain regions from two donors. Using graph genomes constructed from phased genomic sequence data, we compared ChIP-seq signal between alleles at heterozygous variants within each tissue sample from each donor. Comparison of results from different brain regions within donors and the same regions between donors provided measures of allele bias reproducibility. We identified thousands of DNA variants that show reproducible bias in ChIP-seq for at least one TF. We found that alleles that are rarer in the general population were more likely than common alleles to exhibit large biases, and more frequently led to reduced TF binding. Combining ChIP-seq with RNA-seq, we identified TF-allele interaction biases with RNA bias in a phased allele linked to 6,709 eQTL variants identified in GTEx data, 3,309 of which were found in neural contexts. Our results provide insights into the effects of both common and rare variation on gene regulation in the brain. These findings can facilitate mechanistic understanding of cis-regulatory variation associated with biological traits, including disease.
Figures



References
-
- Andersen BB, Korbo L, Pakkenberg B. 1992. A quantitative study of the human cerebellum with unbiased stereological techniques. J Comp Neurol 326: 549–560. - PubMed
-
- Bioconductor Core Team BPMO [Cre. 2017. TxDb.Hsapiens.UCSC.hg38.knownGene. https://bioconductor.org/packages/TxDb.Hsapiens.UCSC.hg38.knownGene (Accessed August 23, 2023).
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
