This is a preprint.
Phosphoglycerate kinase is a central leverage point in Parkinson's Disease driven neuronal metabolic deficits
- PMID: 37873141
- PMCID: PMC10592794
- DOI: 10.1101/2023.10.10.561760
Phosphoglycerate kinase is a central leverage point in Parkinson's Disease driven neuronal metabolic deficits
Update in
-
Phosphoglycerate kinase is a central leverage point in Parkinson's disease-driven neuronal metabolic deficits.Sci Adv. 2024 Aug 23;10(34):eadn6016. doi: 10.1126/sciadv.adn6016. Epub 2024 Aug 21. Sci Adv. 2024. PMID: 39167658 Free PMC article.
Abstract
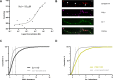
Phosphoglycerate kinase 1 (PGK1), the first ATP producing glycolytic enzyme, has emerged as a therapeutic target for Parkinson's Disease (PD), since a potential enhancer of its activity was reported to significantly lower PD risk. We carried out a suppressor screen of hypometabolic synaptic deficits and demonstrated that PGK1 is a rate limiting enzyme in nerve terminal ATP production. Increasing PGK1 expression in mid-brain dopamine neurons protected against hydroxy-dopamine driven striatal dopamine nerve terminal dysfunction in-vivo and modest changes in PGK1 activity dramatically suppressed hypometabolic synapse dysfunction in vitro. Furthermore, PGK1 is cross-regulated by PARK7 (DJ-1), a PD associated molecular chaperone, and synaptic deficits driven by PARK20 (Synaptojanin-1) can be reversed by increasing local synaptic PGK1 activity. These data indicate that nerve terminal bioenergetic deficits may underly a spectrum of PD susceptibilities and the identification of PGK1 as the limiting enzyme in axonal glycolysis provides a mechanistic underpinning for therapeutic protection.
Figures






References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
