This is a preprint.
Transthalamic input to higher-order cortex selectively conveys state information
- PMID: 37873181
- PMCID: PMC10592671
- DOI: 10.1101/2023.10.08.561424
Transthalamic input to higher-order cortex selectively conveys state information
Update in
-
Higher-order thalamic input to cortex selectively conveys state information.Cell Rep. 2025 Feb 25;44(2):115292. doi: 10.1016/j.celrep.2025.115292. Epub 2025 Feb 11. Cell Rep. 2025. PMID: 39937647 Free PMC article.
Abstract
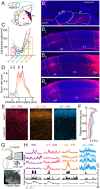
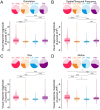
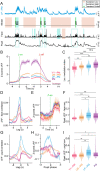
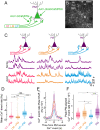
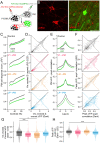
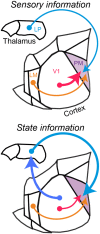
Communication among different neocortical areas is largely thought to be mediated by long-range synaptic interactions between cortical neurons, with the thalamus providing only an initial relay of information from the sensory periphery. Higher-order thalamic nuclei receive strong synaptic inputs from the cortex and send robust projections back to other cortical areas, providing a distinct and potentially critical route for cortico-cortical communication. However, the relative contributions of corticocortical and thalamocortical inputs to higher-order cortical function remain unclear. Using imaging of cortical neurons and projection axon terminals in combination with optogenetic manipulations, we find that the higher-order visual thalamus of mice conveys a specialized stream of information to higher-order visual cortex. Whereas corticocortical projections from lower cortical areas convey robust visual information, higher-order thalamocortical projections convey strong behavioral state information. Together, these findings suggest a key role for higher-order thalamus in providing contextual signals that flexibly modulate sensory processing in higher-order cortex.
Figures
Similar articles
-
Higher-order thalamic input to cortex selectively conveys state information.Cell Rep. 2025 Feb 25;44(2):115292. doi: 10.1016/j.celrep.2025.115292. Epub 2025 Feb 11. Cell Rep. 2025. PMID: 39937647 Free PMC article.
-
DISRUPTION OF TRANSTHALAMIC CIRCUITRY FROM PRIMARY VISUAL CORTEX IMPAIRS VISUAL DISCRIMINATION IN MICE.bioRxiv [Preprint]. 2025 Feb 8:2025.02.07.637190. doi: 10.1101/2025.02.07.637190. bioRxiv. 2025. Update in: J Neurosci. 2025 Mar 26:e0002252025. doi: 10.1523/JNEUROSCI.0002-25.2025. PMID: 39975026 Free PMC article. Updated. Preprint.
-
DISRUPTION OF TRANSTHALAMIC CIRCUITRY FROM PRIMARY VISUAL CORTEX IMPAIRS VISUAL DISCRIMINATION IN MICE.J Neurosci. 2025 Mar 26;45(18):e0002252025. doi: 10.1523/JNEUROSCI.0002-25.2025. Online ahead of print. J Neurosci. 2025. PMID: 40139804
-
Functioning of Circuits Connecting Thalamus and Cortex.Compr Physiol. 2017 Mar 16;7(2):713-739. doi: 10.1002/cphy.c160032. Compr Physiol. 2017. PMID: 28333385 Review.
-
Thalamic relay functions and their role in corticocortical communication: generalizations from the visual system.Neuron. 2002 Jan 17;33(2):163-75. doi: 10.1016/s0896-6273(01)00582-7. Neuron. 2002. PMID: 11804565 Review.
References
-
- Aarts E, Verhage M, Veenvliet JV, Dolan CV, van der Sluis S (2014) A solution to dependency: using multilevel analysis to accommodate nested data. Nat. Neurosci. 17: 491–496. - PubMed
-
- Beltramo R, Scanziani M (2019) A collicular visual cortex: neocortical space for an ancient midbrain visual structure. Science 363: 64–69. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
