This is a preprint.
Exome copy number variant detection, analysis and classification in a large cohort of families with undiagnosed rare genetic disease
- PMID: 37873196
- PMCID: PMC10593084
- DOI: 10.1101/2023.10.05.23296595
Exome copy number variant detection, analysis and classification in a large cohort of families with undiagnosed rare genetic disease
Update in
-
Exome copy number variant detection, analysis, and classification in a large cohort of families with undiagnosed rare genetic disease.Am J Hum Genet. 2024 May 2;111(5):863-876. doi: 10.1016/j.ajhg.2024.03.008. Epub 2024 Apr 1. Am J Hum Genet. 2024. PMID: 38565148 Free PMC article.
Abstract
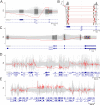
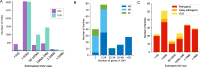
Copy number variants (CNVs) are significant contributors to the pathogenicity of rare genetic diseases and with new innovative methods can now reliably be identified from exome sequencing. Challenges still remain in accurate classification of CNV pathogenicity. CNV calling using GATK-gCNV was performed on exomes from a cohort of 6,633 families (15,759 individuals) with heterogeneous phenotypes and variable prior genetic testing collected at the Broad Institute Center for Mendelian Genomics of the GREGoR consortium. Each family's CNV data was analyzed using the seqr platform and candidate CNVs classified using the 2020 ACMG/ClinGen CNV interpretation standards. We developed additional evidence criteria to address situations not covered by the current standards. The addition of CNV calling to exome analysis identified causal CNVs for 173 families (2.6%). The estimated sizes of CNVs ranged from 293 bp to 80 Mb with estimates that 44% would not have been detected by standard chromosomal microarrays. The causal CNVs consisted of 141 deletions, 15 duplications, 4 suspected complex structural variants (SVs), 3 insertions and 10 complex SVs, the latter two groups being identified by orthogonal validation methods. We interpreted 153 CNVs as likely pathogenic/pathogenic and 20 CNVs as high interest variants of uncertain significance. Calling CNVs from existing exome data increases the diagnostic yield for individuals undiagnosed after standard testing approaches, providing a higher resolution alternative to arrays at a fraction of the cost of genome sequencing. Our improvements to the classification approach advances the systematic framework to assess the pathogenicity of CNVs.
Conflict of interest statement
Declaration of interests H.L.R. has received support from Illumina and Microsoft to support rare disease gene discovery and diagnosis. A.O-D.L. has consulted for Tome Biosciences and Ono Pharma USA Inc. D.G.M is a paid advisor to GlaxoSmithKline, Insitro, Variant Bio and Overtone Therapeutics, and has received research support from AbbVie, Astellas, Biogen, BioMarin, Eisai, Google, Merck, Microsoft, Pfizer, and Sanofi-Genzyme. C.A.W. is a paid advisor to Maze Therapeutics. M.E.T. receives research funding from Microsoft Inc, Illumina Inc and Levo Therapeutics. The remaining authors declare no competing interests.
Figures

References
-
- Zarrei M., MacDonald J.R., Merico D., and Scherer S.W. (2015). A copy number variation map of the human genome. Nat. Rev. Genet. 16, 172–183. - PubMed
-
- Weischenfeldt J., Symmons O., Spitz F., and Korbel J.O. (2013). Phenotypic impact of genomic structural variation: insights from and for human disease. Nat. Rev. Genet. 14, 125–138. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
