This is a preprint.
Ventral hippocampus neurons encode meal-related memory
- PMID: 37873229
- PMCID: PMC10592790
- DOI: 10.1101/2023.10.10.561731
Ventral hippocampus neurons encode meal-related memory
Update in
-
Ventral hippocampus neurons encode meal-related memory.Nat Commun. 2025 Jun 11;16(1):4898. doi: 10.1038/s41467-025-59687-1. Nat Commun. 2025. PMID: 40500290 Free PMC article.
Abstract
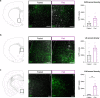
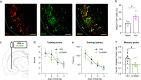
The ability to encode and retrieve meal-related information is critical to efficiently guide energy acquisition and consumption, yet the underlying neural processes remain elusive. Here we reveal that ventral hippocampus (HPCv) neuronal activity dynamically elevates during meal consumption and this response is highly predictive of subsequent performance in a foraging-related spatial memory task. Targeted recombination-mediated ablation of HPCv meal-responsive neurons impairs foraging-related spatial memory without influencing food motivation, anxiety-like behavior, or escape-mediated spatial memory. These HPCv meal-responsive neurons project to the lateral hypothalamic area (LHA) and single-nucleus RNA sequencing and in situ hybridization analyses indicate they are enriched in serotonin 2a receptors (5HT2aR). Either chemogenetic silencing of HPCv-to-LHA projections or intra-HPCv 5HT2aR antagonist yielded foraging-related spatial memory deficits, as well as alterations in caloric intake and the temporal sequence of spontaneous meal consumption. Collective results identify a population of HPCv neurons that dynamically respond to eating to encode meal-related memories.
Conflict of interest statement
CONFLICT OF INTEREST BCR and MRH both receive research funding from Novo Nordisk and Boehringer Ingelheim that was not used in support of these studies. MRH receives research funding from Eli Lilly & Co., Gila Therapeutics, and Pfizer that was not used in support of these studies. MRH is CEO of Cantius Therapeutics, LLC which pursues biological work unrelated to the current study. All other authors declare no competing interests.
Figures





References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
