This is a preprint.
A murine model of Trypanosoma brucei-induced myocarditis and cardiac dysfunction
- PMID: 37873308
- PMCID: PMC10592974
- DOI: 10.1101/2023.10.05.560950
A murine model of Trypanosoma brucei-induced myocarditis and cardiac dysfunction
Update in
-
A murine model of Trypanosoma brucei-induced myocarditis and cardiac dysfunction.Microbiol Spectr. 2025 Feb 4;13(2):e0162324. doi: 10.1128/spectrum.01623-24. Epub 2025 Jan 10. Microbiol Spectr. 2025. PMID: 39791886 Free PMC article.
Abstract
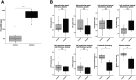
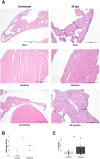
Trypanosoma brucei is a protozoan parasite that causes human and animal African trypanosomiases (HAT and AAT). Cardiac symptoms are commonly reported in HAT patients, and intracardiac parasites with accompanying myocarditis have been observed in both natural hosts and animal models of T. brucei infection. Despite the importance of T. brucei as a cause of cardiac dysfunction and the dramatic socioeconomic impact of African trypanosomiases in sub-Saharan Africa, there are currently no reproducible murine models of T. brucei-associated cardiomyopathy. We present the first clinically relevant, reproducible murine model of cardiac dysfunction in chronic T. brucei infection. Similar to humans, mice showed histological evidence of myocarditis and elevation of serum NT-proBNP with electrocardiographic abnormalities. Serum NT-proBNP levels were elevated prior to the development of severe ventricular dysfunction. On flow cytometry, myocarditis was associated with an increase of most myocardial immune cell populations, including multiple T cell and macrophage subsets, corroborating the notion that T. brucei-associated cardiac damage is an immune-mediated event. This novel mouse model represents a powerful and practical tool to investigate the pathogenesis of T. brucei-mediated heart damage and supports the development of therapeutic options for T. brucei-associated cardiac disease.
Conflict of interest statement
Conflict-of-interest statement The authors have declared that no conflicts of interest exist
Figures




References
-
- Spickler AR. African Animal Trypanosomiasis [Internet]. IO State University Center for Food Security and Public Health. 2018.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
