This is a preprint.
Stem cell-specific ecdysone signaling regulates the development and function of a Drosophila sleep homeostat
- PMID: 37873323
- PMCID: PMC10592846
- DOI: 10.1101/2023.09.29.560022
Stem cell-specific ecdysone signaling regulates the development and function of a Drosophila sleep homeostat
Update in
-
Stem cell-specific ecdysone signaling regulates the development of dorsal fan-shaped body neurons and sleep homeostasis.Curr Biol. 2024 Nov 4;34(21):4951-4967.e5. doi: 10.1016/j.cub.2024.09.020. Epub 2024 Oct 8. Curr Biol. 2024. PMID: 39383867 Free PMC article.
Abstract
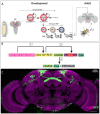
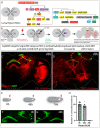
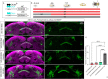
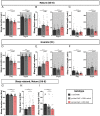
Complex behaviors arise from neural circuits that are assembled from diverse cell types. Sleep is a conserved and essential behavior, yet little is known regarding how the nervous system generates neuron types of the sleep-wake circuit. Here, we focus on the specification of Drosophila sleep-promoting neurons-long-field tangential input neurons that project to the dorsal layers of the fan-shaped body neuropil in the central complex (CX). We use lineage analysis and genetic birth dating to identify two bilateral Type II neural stem cells that generate these dorsal fan-shaped body (dFB) neurons. We show that adult dFB neurons express Ecdysone-induced protein E93, and loss of Ecdysone signaling or E93 in Type II NSCs results in the misspecification of the adult dFB neurons. Finally, we show that E93 knockdown in Type II NSCs affects adult sleep behavior. Our results provide insight into how extrinsic hormonal signaling acts on NSCs to generate neuronal diversity required for adult sleep behavior. These findings suggest that some adult sleep disorders might derive from defects in stem cell-specific temporal neurodevelopmental programs.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures


References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
