This is a preprint.
Methylphenidate alleviates cognitive dysfunction from early Mn exposure: Role of catecholaminergic receptors
- PMID: 37873333
- PMCID: PMC10592804
- DOI: 10.1101/2023.06.27.546786
Methylphenidate alleviates cognitive dysfunction from early Mn exposure: Role of catecholaminergic receptors
Update in
-
Methylphenidate alleviates cognitive dysfunction caused by early manganese exposure: Role of catecholaminergic receptors.Prog Neuropsychopharmacol Biol Psychiatry. 2024 Apr 20;131:110949. doi: 10.1016/j.pnpbp.2024.110949. Epub 2024 Jan 23. Prog Neuropsychopharmacol Biol Psychiatry. 2024. PMID: 38266866
Abstract
Environmental manganese (Mn) exposure is associated with impaired attention and psychomotor functioning, as well as impulsivity/hyperactivity in children and adolescents. We have shown previously that developmental Mn exposure can cause these same dysfunctions in a rat model. Methylphenidate (MPH) lessens impairments in attention, impulse control, and sensorimotor function in children, but it is unknown whether MPH ameliorates these dysfunctions when induced by developmental Mn exposure. Here, we sought to (1) determine whether oral MPH treatment ameliorates the lasting attention and sensorimotor impairments caused by developmental Mn exposure, and (2) elucidate the mechanism(s) of Mn neurotoxicity and MPH effectiveness. Rats were given 50 mg Mn/kg/d orally over PND 1-21 and assessed as adults in a series of attention, impulse control and sensorimotor tasks during oral MPH treatment (0, 0.5, 1.5, or 3.0 mg/kg/d). Subsequently, selective catecholaminergic receptor antagonists were administered to gain insight into the mechanism(s) of action of Mn and MPH. Developmental Mn exposure caused persistent attention and sensorimotor impairments. MPH treatment at 0.5 mg/kg/d completely ameliorated the Mn attentional dysfunction, whereas the sensorimotor deficits were ameliorated by the 3.0 mg/kg/d MPH dose. Notably, the MPH benefit on attention was only apparent after prolonged treatment, while MPH efficacy for the sensorimotor deficits emerged early in treatment. Selectively antagonizing D1, D2, or α2A receptors had no effect on the Mn-induced attentional dysfunction or MPH efficacy in this domain. However, antagonism of D2R attenuated the Mn sensorimotor deficits, whereas the efficacy of MPH to ameliorate those deficits was diminished by D1R antagonism. These findings demonstrate that MPH is effective in alleviating the lasting attention and sensorimotor dysfunction caused by developmental Mn exposure, and they clarify the mechanisms underlying developmental Mn neurotoxicity and MPH efficacy. Given that the cause of attention and psychomotor deficits in children is often unknown, these findings have implications for the treatment of environmentally-induced attentional and psychomotor dysfunction in children more broadly.
Keywords: ADHD; Attention; Manganese; Mechanisms; Methylphenidate; Sensorimotor.
Figures



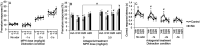
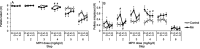

References
-
- Arnsten A.F.T., 2009. Toward a New Understanding of Attention-Deficit Hyperactivity Disorder Pathophysiology An Important Role for Prefrontal Cortex Dysfunction, CNS Drugs. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
