This is a preprint.
Cholinergic modulation of rearing in rats performing a spatial memory task
- PMID: 37873370
- PMCID: PMC10592823
- DOI: 10.1101/2023.10.14.559618
Cholinergic modulation of rearing in rats performing a spatial memory task
Update in
-
Cholinergic modulation of rearing in rats performing a spatial memory task.Eur J Neurosci. 2024 May;59(9):2240-2255. doi: 10.1111/ejn.16248. Epub 2024 Jan 23. Eur J Neurosci. 2024. PMID: 38258622 Free PMC article.
Abstract
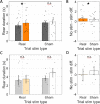
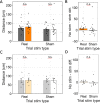
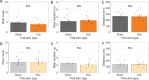
Spatial memory encoding depends in part on cholinergic modulation. How acetylcholine supports spatial memory encoding is not well understood. Prior studies indicate that acetylcholine release is correlated with exploration, including epochs of rearing onto hind legs. Here, to test whether elevated cholinergic tone increases the probability of rearing, we tracked rearing frequency and duration while optogenetically modulating the activity of choline acetyltransferase containing (i.e., acetylcholine producing) neurons of the medial septum in rats performing a spatial working memory task (n = 17 rats). The cholinergic neurons were optogenetically inhibited using halorhodopsin for the duration that rats occupied two of the four open arms during the study phase of an 8-arm radial arm maze win-shift task. Comparing rats' behavior in the two arm types showed that rearing frequency was not changed but the average duration of rearing epochs became significantly longer. This effect on rearing was observed during optogenetic inhibition but not during sham inhibition or in rats that received infusions of a fluorescent reporter virus (i.e., without halorhodopsin; n = 6 rats). Optogenetic inhibition of cholinergic neurons during the pre-trial waiting phase had no significant effect on rearing, indicating a context-specificity of the observed effects. These results are significant in that they indicate that cholinergic neuron activity in the medial septum is correlated with rearing not because it motivates an exploratory state but because it contributes to the processing of information acquired while rearing.
Keywords: Acetylcholine; Exploration; Hippocampus; Medial septum; Optogenetics; Rat; Rearing; Reversible inactivation; Spatial memory.
Conflict of interest statement
Competing Interests The authors have no competing interests to declare.
Figures




References
-
- Abeelen J. H. F. van. (1989). Genetic control of hippocampal cholinergic and dynorphinergic mechanisms regulating novelty-induced exploratory behavior in house mice. Experientia 45, 839–845. - PubMed
-
- Blokland A., Honig W. & Raaijmakers W.G.M. (1992). Effects of intra-hippocampal scopolamine injections in a repeated spatial acquisition task in the rat. Psychopharmacology 109, 373–376. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
