This is a preprint.
Microbiota-dependent indole production is required for the development of collagen-induced arthritis
- PMID: 37873395
- PMCID: PMC10592798
- DOI: 10.1101/2023.10.13.561693
Microbiota-dependent indole production is required for the development of collagen-induced arthritis
Update in
-
Microbiota-dependent indole production stimulates the development of collagen-induced arthritis in mice.J Clin Invest. 2023 Dec 19;134(4):e167671. doi: 10.1172/JCI167671. J Clin Invest. 2023. PMID: 38113112 Free PMC article.
Abstract
Altered tryptophan catabolism has been identified in inflammatory diseases like rheumatoid arthritis (RA) and spondyloarthritis (SpA), but the causal mechanisms linking tryptophan metabolites to disease are unknown. Using the collagen-induced arthritis (CIA) model we identify alterations in tryptophan metabolism, and specifically indole, that correlate with disease. We demonstrate that both bacteria and dietary tryptophan are required for disease, and indole supplementation is sufficient to induce disease in their absence. When mice with CIA on a low-tryptophan diet were supplemented with indole, we observed significant increases in serum IL-6, TNF, and IL-1β; splenic RORγt+CD4+ T cells and ex vivo collagen-stimulated IL-17 production; and a pattern of anti-collagen antibody isotype switching and glycosylation that corresponded with increased complement fixation. IL-23 neutralization reduced disease severity in indole-induced CIA. Finally, exposure of human colon lymphocytes to indole increased expression of genes involved in IL-17 signaling and plasma cell activation. Altogether, we propose a mechanism by which intestinal dysbiosis during inflammatory arthritis results in altered tryptophan catabolism, leading to indole stimulation of arthritis development. Blockade of indole generation may present a novel therapeutic pathway for RA and SpA.
Conflict of interest statement
Conflict-of-interest statement: the authors have declared that no conflict of interest exists.
Figures



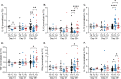


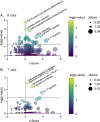
References
-
- Maeda Y., et al. , Dysbiosis Contributes to Arthritis Development via Activation of Autoreactive T Cells in the Intestine. Arthritis & Rheumatology, 2016. 68(11): p. 2646–2661. - PubMed
Publication types
Grants and funding
- IK2 BX006088/BX/BLRD VA/United States
- IK6 BX006475/BX/BLRD VA/United States
- T32 AI007405/AI/NIAID NIH HHS/United States
- R01 AR075033/AR/NIAMS NIH HHS/United States
- P30 AR079369/AR/NIAMS NIH HHS/United States
- T32 AR007534/AR/NIAMS NIH HHS/United States
- I01 BX002182/BX/BLRD VA/United States
- R01 DK095491/DK/NIDDK NIH HHS/United States
- R01 DK104713/DK/NIDDK NIH HHS/United States
- R01 DK103712/DK/NIDDK NIH HHS/United States
- F30 AI174817/AI/NIAID NIH HHS/United States
- R01 DK050189/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
