This is a preprint.
Parkinsonism Sac domain mutation in Synaptojanin-1 affects ciliary properties in iPSC-derived dopaminergic neurons
- PMID: 37873399
- PMCID: PMC10592818
- DOI: 10.1101/2023.10.12.562142
Parkinsonism Sac domain mutation in Synaptojanin-1 affects ciliary properties in iPSC-derived dopaminergic neurons
Update in
-
Parkinsonism Sac domain mutation in Synaptojanin-1 affects ciliary properties in iPSC-derived dopaminergic neurons.Proc Natl Acad Sci U S A. 2024 Apr 23;121(17):e2318943121. doi: 10.1073/pnas.2318943121. Epub 2024 Apr 18. Proc Natl Acad Sci U S A. 2024. PMID: 38635628 Free PMC article.
Abstract
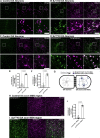
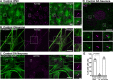
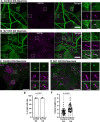
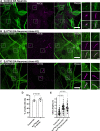
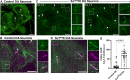
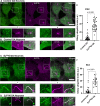
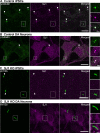
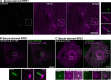
Synaptojanin-1 (SJ1) is a major neuronal-enriched PI(4,5)P2 4- and 5-phosphatase implicated in the shedding of endocytic factors during endocytosis. A mutation (R258Q) that impairs selectively its 4-phosphatase activity causes Parkinsonism in humans and neurological defects in mice (SJ1RQKI mice). Studies of these mice showed, besides an abnormal assembly state of endocytic factors at synapses, the presence of dystrophic nerve terminals selectively in a subset of nigro-striatal dopamine (DA)-ergic axons, suggesting a special lability of DA neurons to the impairment of SJ1 function. Here we have further investigated the impact of SJ1 on DA neurons using iPSC-derived SJ1 KO and SJ1RQKI DA neurons and their isogenic controls. In addition to the expected enhanced clustering of endocytic factors in nerve terminals, we observed in both SJ1 mutant neuronal lines increased cilia length. Further analysis of cilia of SJ1RQDA neurons revealed abnormal accumulation of the Ca2+ channel Cav1.3 and of ubiquitin chains, suggesting an impaired clearing of proteins from cilia which may result from an endocytic defect at the ciliary base, where a focal concentration of SJ1 was observed. We suggest that SJ1 may contribute to the control of ciliary protein dynamics in DA neurons, with implications on cilia-mediated signaling.
Keywords: Primary cilia; calcium signaling; centriole; neurodegenerative disease; ubiquitin.
Figures
Similar articles
-
Parkinsonism Sac domain mutation in Synaptojanin-1 affects ciliary properties in iPSC-derived dopaminergic neurons.Proc Natl Acad Sci U S A. 2024 Apr 23;121(17):e2318943121. doi: 10.1073/pnas.2318943121. Epub 2024 Apr 18. Proc Natl Acad Sci U S A. 2024. PMID: 38635628 Free PMC article.
-
Mutations in Parkinsonism-linked endocytic proteins synaptojanin1 and auxilin have synergistic effects on dopaminergic axonal pathology.NPJ Parkinsons Dis. 2023 Feb 15;9(1):26. doi: 10.1038/s41531-023-00465-5. NPJ Parkinsons Dis. 2023. PMID: 36792618 Free PMC article.
-
Absence of Sac2/INPP5F enhances the phenotype of a Parkinson's disease mutation of synaptojanin 1.Proc Natl Acad Sci U S A. 2020 Jun 2;117(22):12428-12434. doi: 10.1073/pnas.2004335117. Epub 2020 May 18. Proc Natl Acad Sci U S A. 2020. PMID: 32424101 Free PMC article.
-
Synaptic dysfunction in Parkinson's disease.Adv Exp Med Biol. 2012;970:553-72. doi: 10.1007/978-3-7091-0932-8_24. Adv Exp Med Biol. 2012. PMID: 22351072 Review.
-
Converging roles of ion channels, calcium, metabolic stress, and activity pattern of Substantia nigra dopaminergic neurons in health and Parkinson's disease.J Neurochem. 2016 Oct;139 Suppl 1(Suppl Suppl 1):156-178. doi: 10.1111/jnc.13572. Epub 2016 Mar 23. J Neurochem. 2016. PMID: 26865375 Free PMC article. Review.
References
-
- Alessi D. R. and Sammler E. (2018). “LRRK2 kinase in Parkinson’s disease.” Science 360(6384): 36–37. - PubMed
-
- Bartolome A., Heiby J. C., Dau T., Fraia D. D., Heinze I., Kirkpatrick J. M. and Ori A. (2022). “ProteasomeID: quantitative mapping of proteasome interactomes and substrates for in vitro and in vivo studies.” bioRxiv: 2022.2008.2009.503299.
-
- Bielas S. L., Silhavy J. L., Brancati F., Kisseleva M. V., Al-Gazali L., Sztriha L., Bayoumi R. A., Zaki M. S., Abdel-Aleem A., Rosti R. O., Kayserili H., Swistun D., Scott L. C., Bertini E., Boltshauser E., Fazzi E., Travaglini L., Field S. J., Gayral S., Jacoby M., Schurmans S., Dallapiccola B., Majerus P. W., Valente E. M. and Gleeson J. G. (2009). “Mutations in INPP5E, encoding inositol polyphosphate-5-phosphatase E, link phosphatidyl inositol signaling to the ciliopathies.” Nat Genet 41(9): 1032–1036. - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
