This is a preprint.
Growth Dynamics of Ductal Carcinoma in Situ Recapitulate Normal Breast Development
- PMID: 37873488
- PMCID: PMC10592867
- DOI: 10.1101/2023.10.01.560370
Growth Dynamics of Ductal Carcinoma in Situ Recapitulate Normal Breast Development
Abstract
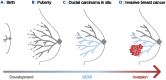
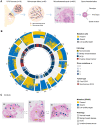
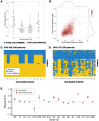
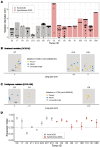
Ductal carcinoma in situ (DCIS) and invasive breast cancer share many morphologic, proteomic, and genomic alterations. Yet in contrast to invasive cancer, many DCIS tumors do not progress and may remain indolent over decades. To better understand the heterogenous nature of this disease, we reconstructed the growth dynamics of 18 DCIS tumors based on the geo-spatial distribution of their somatic mutations. The somatic mutation topographies revealed that DCIS is multiclonal and consists of spatially discontinuous subclonal lesions. Here we show that this pattern of spread is consistent with a new 'Comet' model of DCIS tumorigenesis, whereby multiple subclones arise early and nucleate the buds of the growing tumor. The discontinuous, multiclonal growth of the Comet model is analogous to the branching morphogenesis of normal breast development that governs the rapid expansion of the mammary epithelium during puberty. The branching morphogenesis-like dynamics of the proposed Comet model diverges from the canonical model of clonal evolution, and better explains observed genomic spatial data. Importantly, the Comet model allows for the clinically relevant scenario of extensive DCIS spread, without being subjected to the selective pressures of subclone competition that promote the emergence of increasingly invasive phenotypes. As such, the normal cell movement inferred during DCIS growth provides a new explanation for the limited risk of progression in DCIS and adds biologic rationale for ongoing clinical efforts to reduce DCIS overtreatment.
Conflict of interest statement
COMPETING INTERESTS The authors declare no competing interests.
Figures


References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
