This is a preprint.
Effects of COVID-19 mRNA vaccination on HIV viremia and reservoir size
- PMID: 37873490
- PMCID: PMC10593027
- DOI: 10.1101/2023.10.08.23296718
Effects of COVID-19 mRNA vaccination on HIV viremia and reservoir size
Update in
-
Effects of COVID-19 mRNA vaccination on HIV viremia and reservoir size.AIDS. 2024 Jul 1;38(8):1120-1130. doi: 10.1097/QAD.0000000000003841. Epub 2024 Jan 22. AIDS. 2024. PMID: 38224350 Free PMC article.
Abstract
Objective: The immunogenic nature of COVID-19 mRNA vaccines led to some initial concern that these could stimulate the HIV reservoir. We analyzed changes in plasma HIV loads (pVL) and reservoir size following COVID-19 mRNA vaccination in 62 people with HIV (PWH) receiving antiretroviral therapy (ART), and analyzed province-wide trends in pVL before and after the mass vaccination campaign.
Design: Longitudinal observational cohort and province-wide analysis.
Methods: 62 participants were sampled pre-vaccination, and one month after their first and second COVID-19 immunizations. Vaccine-induced anti-SARS-CoV-2-Spike antibodies in serum were measured using the Roche Elecsys Anti-S assay. HIV reservoirs were quantified using the Intact Proviral DNA Assay; pVL were measured using the cobas 6800 (LLOQ:20 copies/mL). The province-wide analysis included all 290,401 pVL performed in British Columbia, Canada between 2012-2022.
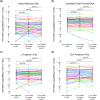
Results: Pre-vaccination, the median intact reservoir size was 77 (IQR:20-204) HIV copies/million CD4+ T-cells, compared to 74 (IQR:27-212) and 65 (IQR:22-174) post-first and -second dose, respectively (all comparisons p>0.07). Pre-vaccination, 82% of participants had pVL<20 copies/mL (max:110 copies/mL), compared to 79% post-first dose (max:183 copies/mL) and 85% post-second dose (max:79 copies/mL) (p>0.4). The magnitude of the vaccine-elicited anti-SARS-CoV-2-Spike antibody response did not correlate with changes in reservoir size nor detectable pVL frequency (p>0.6). We found no evidence linking the COVID-19 mass vaccination campaign to population-level increases in detectable pVL frequency among all PWH in the province, nor among those who maintained pVL suppression on ART.
Conclusion: We found no evidence that COVID-19 mRNA vaccines induced changes in HIV reservoir size nor plasma viremia.
Keywords: COVID-19 vaccine; HIV; IPDA; mRNA; plasma viral load; reservoir size.
Figures



References
-
- Geretti AM, Stockdale AJ, Kelly SH, Cevik M, Collins S, Waters L, et al. Outcomes of Coronavirus Disease 2019 (COVID-19) Related Hospitalization Among People With Human Immunodeficiency Virus (HIV) in the ISARIC World Health Organization (WHO) Clinical Characterization Protocol (UK): A Prospective Observational Study. Clin Infect Dis 2020; 73:e2095–e2106. - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
