Delivery of Plasmid DNA by Ionizable Lipid Nanoparticles to Induce CAR Expression in T Cells
- PMID: 37873551
- PMCID: PMC10590593
- DOI: 10.2147/IJN.S424723
Delivery of Plasmid DNA by Ionizable Lipid Nanoparticles to Induce CAR Expression in T Cells
Abstract
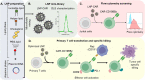
Introduction: Chimeric antigen receptor (CAR) cell therapy represents a hallmark in cancer immunotherapy, with significant clinical results in the treatment of hematological tumors. However, current approved methods to engineer T cells to express CAR use viral vectors, which are integrative and have been associated with severe adverse effects due to constitutive expression of CAR. In this context, non-viral vectors such as ionizable lipid nanoparticles (LNPs) arise as an alternative to engineer CAR T cells with transient expression of CAR.
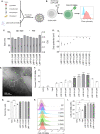
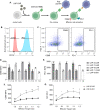
Methods: Here, we formulated a mini-library of LNPs to deliver pDNA to T cells by varying the molar ratios of excipient lipids in each formulation. LNPs were characterized and screened in vitro using a T cell line (Jurkat). The optimized formulation was used ex vivo to engineer T cells derived from human peripheral blood mononuclear cells (PBMCs) for the expression of an anti-CD19 CAR (CAR-CD19BBz). The effectiveness of these CAR T cells was assessed in vitro against Raji (CD19+) cells.
Results: LNPs formulated with different molar ratios of excipient lipids efficiently delivered pDNA to Jurkat cells with low cytotoxicity compared to conventional transfection methods, such as electroporation and lipofectamine. We show that CAR-CD19BBz expression in T cells was transient after transfection with LNPs. Jurkat cells transfected with our top-performing LNPs underwent activation when exposed to CD19+ target cells. Using our top-performing LNP-9-CAR, we were able to engineer human primary T cells to express CAR-CD19BBz, which elicited significant specific killing of CD19+ target cells in vitro.
Conclusion: Collectively, our results show that LNP-mediated delivery of pDNA is a suitable method to engineer human T cells to express CAR, which holds promise for improving the production methods and broader application of this therapy in the future.
Keywords: CAR T cells; cancer immunotherapy; cell engineering; lipid nanoparticles; pDNA delivery.
© 2023 Prazeres et al.
Conflict of interest statement
Mr Pedro Henrique Dias Moura Prazeres, Mrs Heloísa Ferreira and Prof. Dr Pedro Pires Goulart Guimaraes report a patent BR1020230120431 pending. The authors report no other conflicts of interest in this work.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

