Enpatoran in COVID-19 pneumonia: Safety and efficacy results from a phase II randomized trial
- PMID: 37873555
- PMCID: PMC10719456
- DOI: 10.1111/cts.13658
Enpatoran in COVID-19 pneumonia: Safety and efficacy results from a phase II randomized trial
Abstract
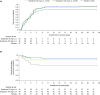
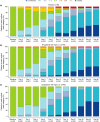
Enpatoran is a selective inhibitor of toll-like receptors 7 and 8 (TLR7/8) that potentially targets pro-inflammatory pathways induced by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). A phase II study conducted in Brazil, the Philippines, and the USA during the early pandemic phase assessed the safety and efficacy of enpatoran in patients hospitalized with COVID-19 pneumonia (NCT04448756). A total of 149 patients, who scored 4 on the World Health Organization's (WHO) 9-point ordinal severity scale, were randomized 1:1:1 and received enpatoran 50 mg (n = 54) or 100 mg (n = 46), or placebo (n = 49) twice daily (b.i.d.) for 14 days plus standard of care. The primary objectives were safety and time to recovery (WHO 9-point scale ≤3). Clinical deterioration (WHO 9-point scale ≥ 5) was a key secondary objective. Treatment-emergent adverse events (TEAEs) were comparable across groups (56.5%-63.0%). Treatment-related TEAEs were numerically higher with enpatoran 50 mg (14.8%) than 100 mg (10.9%) or placebo (8.2%). Serious TEAEs were numerically lower with enpatoran (50 mg 9.3%, 100 mg 2.2%) than placebo (18.4%). The primary efficacy objective was not met; median time to recovery was 3.4-3.9 days across groups, with placebo-treated patients recovering on average faster than anticipated. Clinical deterioration event-free rates up to Day 7 were 90.6%, 95.6%, and 81.6% with enpatoran 50 mg, 100 mg, and placebo, respectively. Enpatoran was well tolerated by patients acutely ill and hospitalized with COVID-19 pneumonia. Positive signals in some secondary end points suggested potential beneficial effects, supporting further evaluation of enpatoran in patients with hyperinflammation due to infection or autoimmunity.
© 2023 Merck KGaA and The Authors. Clinical and Translational Science published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
F.M., J.Sh., and A.H.K. are employees of EMD Serono, L.K.‐S. is an employee of the healthcare business of Merck KGaA, Darmstadt, Germany, and S.R. is an employee of Ares Trading SA, Eysins, Switzerland, an affiliate of Merck KGaA, Darmstadt, Germany. D.Y. and M.K. were employees of EMD Serono and Merck Serono Ltd., Feltham, UK, an affiliate of Merck KGaA, Darmstadt, Germany, respectively, at the time of the study. J.E.M. has served as a consultant for EMD Serono.
Figures


References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

