AZD9567 versus prednisolone in patients with active rheumatoid arthritis: A phase IIa, randomized, double-blind, efficacy, and safety study
- PMID: 37873558
- PMCID: PMC10719483
- DOI: 10.1111/cts.13624
AZD9567 versus prednisolone in patients with active rheumatoid arthritis: A phase IIa, randomized, double-blind, efficacy, and safety study
Abstract
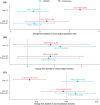
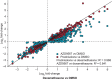
Oral corticosteroid use is limited by side effects, some caused by off-target actions on the mineralocorticoid receptor that disrupt electrolyte balance. AZD9567 is a selective, nonsteroidal glucocorticoid receptor modulator. The efficacy, safety, and tolerability of AZD9567 and prednisolone were assessed in a phase IIa study. Anti-inflammatory mechanism of action was also evaluated in vitro in monocytes from healthy donors. In this randomized, double-blind, parallel-group, multicenter study, patients with active rheumatoid arthritis were randomized 1:1 to AZD9567 40 mg or prednisolone 20 mg once daily orally for 14 days. The primary end point was change from baseline in DAS28-CRP at day 15. Secondary end points included components of DAS28-CRP, American College of Rheumatology (ACR) response criteria (ACR20, ACR50, and ACR70), and safety end points, including serum electrolytes. Overall, 21 patients were randomized to AZD9567 (n = 11) or prednisolone (n = 10), and all completed the study. As anticipated, AZD9567 had a similar efficacy profile to prednisolone, with no clinically meaningful (i.e., >1.0) difference in change from baseline to day 15 in DAS28-CRP between AZD9567 and prednisolone (least-squares mean difference: 0.47, 95% confidence interval: -0.49 to 1.43). Similar results were observed for the secondary efficacy end points. In vitro transcriptomic analysis showed that anti-inflammatory responses were similar for AZD9567, prednisolone, and dexamethasone. Unlike prednisolone, AZD9567 had no effect on the serum sodium:potassium ratio. The safety profile was not different from that of prednisolone. Larger studies of longer duration are required to determine whether AZD9567 40 mg may in the future be an alternative to prednisolone in patients with inflammatory disease.
© 2023 The Authors. Clinical and Translational Science published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
J.M.vL. has received grants from AstraZeneca, MSD, Roche, and Thermo Fisher, and honoraria from AbbVie, Arxx Therapeutics, Boehringer Ingelheim, Galapagos, Gesynta, Leadiant, Magenta, and Sanofi Genzyme. M.S.‐K. received a student grant from AstraZeneca. A.L., J.A., G.B., K.E., L.Ö., B.R.A., I.Di., P.B., D.E., I.Da., C.A., M.G.B., S.N., A.P., S.P., S.S., P.S., and C.K. are employees of, and may own shares in, AstraZeneca.
Figures


References
-
- Smolen JS, Landewé RBM, Bijlsma JWJ, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease‐modifying antirheumatic drugs: 2019 update. Ann Rheum Dis. 2020;79:685‐699. - PubMed
-
- Singh JA, Saag KG, Bridges SL Jr, et al. 2015 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol. 2016;68:1‐26. - PubMed
-
- McKay LI, Cidlowski JA. Physiologic and pharmacologic effects of corticosteroids. In: Kufe DW, Pollock RE, Weichselbaum RR, eds. Holland‐Frei Cancer Medicine. 6th ed. BC Decker; 2003.
-
- Clark AR, Belvisi MG. Maps and legends: the quest for dissociated ligands of the glucocorticoid receptor. Pharmacol Ther. 2012;134:54‐67. - PubMed
-
- Ripa L, Edman K, Dearman M, et al. Discovery of a novel oral glucocorticoid receptor modulator (AZD9567) with improved side effect profile. J Med Chem. 2018;61:1785‐1799. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

