Multiplex Bioanalytical Methods for Comprehensive Characterization and Quantification of the Unique Complementarity-Determining-Region Deamidation of MEDI7247, an Anti-ASCT2 Pyrrolobenzodiazepine Antibody-Drug Conjugate
- PMID: 37873863
- PMCID: PMC10594446
- DOI: 10.3390/antib12040066
Multiplex Bioanalytical Methods for Comprehensive Characterization and Quantification of the Unique Complementarity-Determining-Region Deamidation of MEDI7247, an Anti-ASCT2 Pyrrolobenzodiazepine Antibody-Drug Conjugate
Abstract
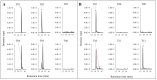
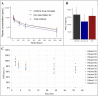
Deamidation, a common post-translational modification, may impact multiple physiochemical properties of a therapeutic protein. MEDI7247, a pyrrolobenzodiazepine (PBD) antibody-drug conjugate (ADC), contains a unique deamidation site, N102, located within the complementarity-determining region (CDR), impacting the affinity of MEDI7247 to its target. Therefore, it was necessary to monitor MEDI7247 deamidation status in vivo. Due to the low dose, a sensitive absolute quantification method using immunocapture coupled with liquid chromatography-tandem mass spectrometry (LBA-LC-MS/MS) was developed and qualified. We characterized the isomerization via Electron-Activated Dissociation (EAD), revealing that deamidation resulted in iso-aspartic acid. The absolute quantification of deamidation requires careful assay optimization in order not to perturb the balance of the deamidated and nondeamidated forms. Moreover, the selection of capture reagents essential for the correct quantitative assessment of deamidation was evaluated. The final assay was qualified with 50 ng/mL LLOQ for ADC for total and nondeamidated antibody quantification, with qualitative monitoring of the deamidated antibody. The impact of deamidation on the pharmacokinetic characteristics of MEDI7247 from clinical trial NCT03106428 was analyzed, revealing a gradual reduction in the nondeamidated form of MEDI7247 in vivo. Careful quantitative biotransformation analyses of complex biotherapeutic conjugates help us understand changes in product PTMs after administration, thus providing a more complete view of in vivo pharmacology.
Keywords: antibody–drug conjugate (ADC); biotransformation; deamidation; hybrid liquid chromatography–mass spectrometry (LC-MS) assay; post-translational modification (PTM).
Conflict of interest statement
All authors (Y.H., J.Y., R.M., R.J.K., K.B., M.C., P.H., N.d.M., D.L., L.K.R., M.L. and A.I.R.) performed work within this manuscript while employed by the company AstraZeneca. The authors’ employment and stock investments in AstraZeneca do not affect the authenticity and objectivity of the experimental results detailed in this manuscript.
Figures





References
-
- Hassanein M., Hoeksema M.D., Shiota M., Qian J., Harris B.K., Chen H., Clark J.E., Alborn W.E., Eisenberg R., Massion P.P. SLC1A5 mediates glutamine transport required for lung cancer cell growth and survival. Clin. Cancer Res. 2013;19:560–570. doi: 10.1158/1078-0432.CCR-12-2334. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

