Protocol of a Phase II Randomized, Multi-Center, Double-Blind, Placebo-Controlled Trial of S-Adenosyl Methionine in Participants with Mild Cognitive Impairment or Dementia Due to Alzheimer's Disease
- PMID: 37874102
- PMCID: PMC10186290
- DOI: 10.14283/jpad.2023.55
Protocol of a Phase II Randomized, Multi-Center, Double-Blind, Placebo-Controlled Trial of S-Adenosyl Methionine in Participants with Mild Cognitive Impairment or Dementia Due to Alzheimer's Disease
Abstract
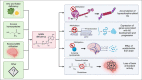
Background: S-adenosyl methionine (SAMe) is a pivotal metabolite in multiple pathways required for neuronal homeostasis, several of which are compromised in Alzheimer's disease (AD). Correction of the SAMe deficiency that is characteristic of the AD brain may attenuate or prevent pathological processes driving AD-associated neurodegeneration including aberrant tau hyperphosphorylation and DNA hypomethylation.
Objectives: The primary aim is to test the hypothesis that daily treatment with 400 mg oral SAMe for 180 days will lead to a greater reduction from baseline in plasma levels of p-tau181 compared to placebo in patients with mild cognitive impairment or dementia due to AD.
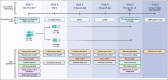
Design, setting, participants: This is a phase II, randomized, multi-center, double-blind, placebo-controlled trial among 60 participants with mild cognitive impairment or dementia due to AD. Participants will be randomized in a 1:1 ratio to receive either SAMe or matching placebo, to be taken as an adjunct to their AD standard of care.
Measurements and results: The primary outcome is change in plasma p-tau181 concentration between baseline and following 180 days of treatment, which will be compared between the active and placebo group. Secondary outcomes are the safety of SAMe administration (incidence of serious adverse events), change from baseline in cognitive performance (as measured by the Repeatable Battery for the Assessment of Neuropsychological Status), and epigenetic changes in DNA methylation.
Conclusion: Demonstration of effective and safe lowering of plasma p-tau181 with SAMe in this phase II trial would pave the way for an exciting field of translational research and a larger phase III trial.
Keywords: Alzheimer’s disease; DNA methylation; S-adenosyl methionine; dementia; tau hyperphosphorylation.
Conflict of interest statement
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Figures

Similar articles
-
Donepezil for dementia due to Alzheimer's disease.Cochrane Database Syst Rev. 2018 Jun 18;6(6):CD001190. doi: 10.1002/14651858.CD001190.pub3. Cochrane Database Syst Rev. 2018. PMID: 29923184 Free PMC article.
-
Nimodipine for primary degenerative, mixed and vascular dementia.Cochrane Database Syst Rev. 2001;(1):CD000147. doi: 10.1002/14651858.CD000147. Cochrane Database Syst Rev. 2001. Update in: Cochrane Database Syst Rev. 2002;(3):CD000147. doi: 10.1002/14651858.CD000147. PMID: 11279679 Updated.
-
Selegiline for Alzheimer's disease.Cochrane Database Syst Rev. 2003;(1):CD000442. doi: 10.1002/14651858.CD000442. Cochrane Database Syst Rev. 2003. PMID: 12535396
-
Galantamine for Alzheimer's disease.Cochrane Database Syst Rev. 2002;(3):CD001747. doi: 10.1002/14651858.CD001747. Cochrane Database Syst Rev. 2002. Update in: Cochrane Database Syst Rev. 2004 Oct 18;(4):CD001747. doi: 10.1002/14651858.CD001747.pub2. PMID: 12137632 Updated.
-
Folic acid with or without vitamin B12 for cognition and dementia.Cochrane Database Syst Rev. 2003;(4):CD004514. doi: 10.1002/14651858.CD004514. Cochrane Database Syst Rev. 2003. Update in: Cochrane Database Syst Rev. 2008 Oct 08;(4):CD004514. doi: 10.1002/14651858.CD004514.pub2. PMID: 14584018 Updated.
Cited by
-
Mitochondrial Targeting against Alzheimer's Disease: Lessons from Hibernation.Cells. 2023 Dec 20;13(1):12. doi: 10.3390/cells13010012. Cells. 2023. PMID: 38201215 Free PMC article. Review.
-
Natural sulfur compounds in mental health and neurological disorders: insights from observational and intervention studies.Front Nutr. 2025 Apr 9;12:1534000. doi: 10.3389/fnut.2025.1534000. eCollection 2025. Front Nutr. 2025. PMID: 40271431 Free PMC article. Review.
References
-
- Ittner L M, Ke Y D, Delerue F, Bi M, Gladbach A, Van Eersel J, Wölfing H, Chieng B C, Christie M J, Napier I A, Eckert A, Staufenbiel M, Hardeman E, Götz J. Dendritic Function of Tau Mediates Amyloid-β Toxicity in Alzheimer’s Disease Mouse Models. Cell. 2010;142(3):387–397. doi: 10.1016/j.cell.2010.06.036. - DOI - PubMed
-
- Olsson B, Lautner R, Andreasson U, Öhrfelt A, Portelius E, Bjerke M, Hölttä M, Rosén C, Olsson C, Strobel G, Wu E, Dakin K, Petzold M, Blennow K, Zetterberg H. CSF and blood biomarkers for the diagnosis of Alzheimer’s disease: a systematic review and meta-analysis. The Lancet Neurology. 2016;15(7):673–684. doi: 10.1016/S1474-4422(16)00070-3. - DOI - PubMed
-
- Gawryluk J W, Wang J-F, Andreazza A C, Shao L, Young L T. Decreased levels of glutathione, the major brain antioxidant, in post-mortem prefrontal cortex from patients with psychiatric disorders. International Journal of Neuropsychopharmacology. 2011;14(1):123–130. doi: 10.1017/S1461145710000805. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
