The directed evolution of NDM-1
- PMID: 37874296
- PMCID: PMC10649027
- DOI: 10.1128/aac.00714-23
The directed evolution of NDM-1
Abstract
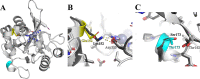
β-Lactam antibiotics are among the most frequently prescribed therapeutic agents. A common mechanism of resistance toward β-lactam antibiotics is the production of β-lactamases. These enzymes are capable of hydrolyzing the β-lactam bond, rendering the drug inactive. Among the four described classes, the metallo- β-lactamases (MBLs, class B) employ one or two zinc ions in the active site for catalysis. One of the three most clinically relevant MBLs is New Delhi Metallo- β-Lactamase (NDM-1). The current study sought to investigate the in vitro protein evolution of NDM-1 β-lactamase using error-prone polymerase chain reaction. Evaluation revealed that variants were not found to confer higher levels of resistance toward meropenem based on amino acid substitutions. Thus, we postulate that increases in transcription or changes in zinc transport may be clinically more relevant to meropenem resistance than amino acid substitutions.
Keywords: NDM-1; antibiotic resistance; antimicrobial resistance; beta-lactamases; beta-lactams; drug resistance evolution.
Conflict of interest statement
Tthe authors declare no conflict of interest.
Figures

References
-
- Cheng Z, Thomas PW, Ju L, Bergstrom A, Mason K, Clayton D, Miller C, Bethel CR, VanPelt J, Tierney DL, Page RC, Bonomo RA, Fast W, Crowder MW. 2018. Evolution of new Delhi Metallo-β-Lactamase (NDM) in the clinic: effects of NDM mutations on stability, zinc affinity, and mono-zinc activity. J Biol Chem 293:12606–12618. doi:10.1074/jbc.RA118.003835 - DOI - PMC - PubMed
-
- Cheng Z, Bethel CR, Thomas PW, Shurina BA, Alao J-P, Thomas CA, Yang K, Marshall SH, Zhang H, Sturgill AM, Kravats AN, Page RC, Fast W, Bonomo RA, Crowder MW. 2021. Carbapenem use is driving the evolution of Imipenemase 1 variants. Antimicrob Agents Chemother 65:1–19. doi:10.1128/AAC.01714-20 - DOI - PMC - PubMed
-
- Antimicrobial resistance: Tackling a crisis for the health and wealth of nations.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

