Mechanistic Analysis of Stereodivergent Nitroalkane Cyclopropanation Catalyzed by Nonheme Iron Enzymes
- PMID: 37874539
- PMCID: PMC10725191
- DOI: 10.1021/jacs.3c08413
Mechanistic Analysis of Stereodivergent Nitroalkane Cyclopropanation Catalyzed by Nonheme Iron Enzymes
Abstract
BelL and HrmJ are α-ketoglutarate-dependent nonheme iron enzymes that catalyze the oxidative cyclization of 6-nitronorleucine, resulting in the formation of two diastereomeric 3-(2-nitrocyclopropyl)alanine (Ncpa) products containing trans-cyclopropane rings with (1'R,2'R) and (1'S,2'S) configurations, respectively. Herein, we investigate the catalytic mechanism and stereodivergency of the cyclopropanases. The results suggest that the nitroalkane moiety of the substrate is first deprotonated to produce the nitronate form. Spectroscopic analyses and biochemical assays with substrates and analogues indicate that an iron(IV)-oxo species abstracts proS-H from C4 to initiate intramolecular C-C bond formation. A hydroxylation intermediate is unlikely to be involved in the cyclopropanation reaction. Additionally, a genome mining approach is employed to discover new homologues that perform the cyclopropanation of 6-nitronorleucine to generate cis-configured Ncpa products with (1'R,2'S) or (1'S,2'R) stereochemistries. Sequence and structure comparisons of these cyclopropanases enable us to determine the amino acid residues critical for controlling the stereoselectivity of cyclopropanation.
Figures
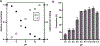

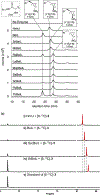


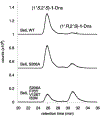

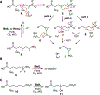
References
-
- Wessjohann LA; Brandt W; Thiemann T, Biosynthesis and Metabolism of Cyclopropane Rings in Natural Compounds. Chemical Reviews 2003, 103 (4), 1625–1648. - PubMed
-
- Ma S; Mandalapu D; Wang S; Zhang Q, Biosynthesis of cyclopropane in natural products. Natural Product Reports 2022, 39 (5), 926–945. - PubMed
-
- Talele TT, The “Cyclopropyl Fragment” is a Versatile Player that Frequently Appears in Preclinical/Clinical Drug Molecules. Journal of Medicinal Chemistry 2016, 59 (19), 8712–8756. - PubMed
-
- Asai A; Hasegawa A; Ochiai K; Yamashita Y; Mizukami T, Belactosin A, a Novel Antituor Antibiotic Acting on Cyclin/CDK Mediated Cell Cycle Regulation, Produced by Streptomyces sp. The Journal of antibiotics 2000, 53 (1), 81–83. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

