Weight Loss-Independent Effect of Liraglutide on Insulin Sensitivity in Individuals With Obesity and Prediabetes
- PMID: 37874653
- PMCID: PMC10784656
- DOI: 10.2337/db23-0356
Weight Loss-Independent Effect of Liraglutide on Insulin Sensitivity in Individuals With Obesity and Prediabetes
Abstract
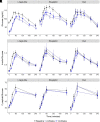
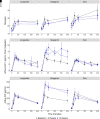
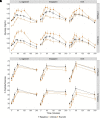
Metabolic effects of glucagon-like peptide 1 (GLP-1) receptor agonists are confounded by weight loss and not fully recapitulated by increasing endogenous GLP-1. We tested the hypothesis that GLP-1 receptor (GLP-1R) agonists exert weight loss-independent, GLP-1R-dependent effects that differ from effects of increasing endogenous GLP-1. Individuals with obesity and prediabetes were randomized to receive for 14 weeks the GLP-1R agonist liraglutide, a hypocaloric diet, or the dipeptidyl peptidase 4 (DPP-4) inhibitor sitagliptin. The GLP-1R antagonist exendin(9-39) and placebo were administered in a two-by-two crossover study during mixed-meal tests. Liraglutide and diet, but not sitagliptin, caused weight loss. Liraglutide improved insulin sensitivity measured by HOMA for insulin resistance (HOMA-IR), the updated HOMA model (HOMA2), and the Matsuda index after 2 weeks, prior to weight loss. Liraglutide decreased fasting and postprandial glucose levels, and decreased insulin, C-peptide, and fasting glucagon levels. In contrast, diet-induced weight loss improved insulin sensitivity by HOMA-IR and HOMA2, but not the Matsuda index, and did not decrease glucose levels. Sitagliptin increased endogenous GLP-1 and GIP values without altering insulin sensitivity or fasting glucose levels, but decreased postprandial glucose and glucagon levels. Notably, sitagliptin increased GIP without altering weight. Acute GLP-1R antagonism increased glucose levels in all groups, increased the Matsuda index and fasting glucagon level during liraglutide treatment, and increased endogenous GLP-1 values during liraglutide and sitagliptin treatments. Thus, liraglutide exerts rapid, weight loss-independent, GLP-1R-dependent effects on insulin sensitivity that are not achieved by increasing endogenous GLP-1.
Article highlights: Metabolic benefits of glucagon-like peptide 1 (GLP-1) receptor agonists are confounded by weight loss and are not fully achieved by increasing endogenous GLP-1 through dipeptidyl peptidase 4 (DPP-4) inhibition. We investigated weight loss-independent, GLP-1 receptor (GLP-1R)-dependent metabolic effects of liraglutide versus a hypocaloric diet or the DPP-4 inhibitor sitagliptin. GLP-1R antagonism with exendin(9-39) was used to assess GLP-1R-dependent effects during mixed meals. Liraglutide improved insulin sensitivity and decreased fasting and postprandial glucose prior to weight loss, and these benefits were reversed by exendin(9-39). GLP-1R agonists exert rapid, weight loss-independent, GLP-1R-dependent effects on insulin sensitivity not achieved by increasing endogenous GLP-1.
© 2023 by the American Diabetes Association.
Conflict of interest statement
Figures


References
-
- Nauck MA, Meier JJ, Cavender MA, Abd El Aziz M, Drucker DJ. Cardiovascular actions and clinical outcomes with glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors. Circulation 2017;136:849–870 - PubMed
-
- Kim SH, Abbasi F, Nachmanoff C, et al. Effect of the glucagon-like peptide-1 analogue liraglutide versus placebo treatment on circulating proglucagon-derived peptides that mediate improvements in body weight, insulin secretion and action: a randomized controlled trial. Diabetes Obes Metab 2021;23:489–498 - PMC - PubMed
-
- Vanderheiden A, Harrison LB, Warshauer JT, et al. Mechanisms of action of liraglutide in patients with type 2 diabetes treated with high-dose insulin. J Clin Endocrinol Metab 2016;101:1798–1806 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

