Mutant PIK3CA is a targetable driver alteration in histiocytic neoplasms
- PMID: 37874915
- PMCID: PMC10711187
- DOI: 10.1182/bloodadvances.2022009349
Mutant PIK3CA is a targetable driver alteration in histiocytic neoplasms
Abstract
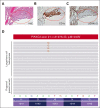
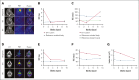
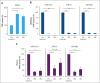
Langerhans cell histiocytosis (LCH) is an inflammatory myeloid neoplasm characterized by the accumulation of clonal mononuclear phagocyte system cells expressing CD1a and CD207. In the past decade, molecular profiling of LCH as well as other histiocytic neoplasms demonstrated that these diseases are driven by MAPK activating alterations, with somatic BRAFV600E mutations in >50% of patients with LCH, and clinical inhibition of MAPK signaling has demonstrated remarkable clinical efficacy. At the same time, activating alterations in kinase-encoding genes, such as PIK3CA, ALK, RET, and CSF1R, which can activate mitogenic pathways independent from the MAPK pathway, have been reported in a subset of histiocytic neoplasms with anecdotal evidence of successful targeted treatment of histiocytoses harboring driver alterations in RET, ALK, and CSF1R. However, evidence supporting the biological consequences of expression of PIK3CA mutations in hematopoietic cells has been lacking, and whether targeted inhibition of PI3K is clinically efficacious in histiocytic neoplasms is unknown. Here, we provide evidence that activating mutations in PIK3CA can drive histiocytic neoplasms in vivo using a conditional knockin mouse expressing mutant PIK3CAH1047R in monocyte/dendritic cell progenitors. In parallel, we demonstrate successful treatment of PIK3CA-mutated, multisystemic LCH using alpelisib, an inhibitor of the alpha catalytic subunit of PI3K. Alpelisib demonstrated a tolerable safety profile at a dose of 750 mg per week and clinical and metabolic complete remission in a patient with PIK3CA-mutated LCH. These data demonstrate PIK3CA as a targetable noncanonical driver of LCH and underscore the importance of mutational analysis-based personalized treatment in histiocytic neoplasms.
© 2023 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: O.A.-W. has served as a consultant for H3 Biomedicine, Foundation Medicine Inc, Merck, Janssen, and Loxo Oncology/Lilly; is on the scientific advisory board of Envisagenics Inc and Harmonic Discovery Inc; and has received prior research funding from H3 Biomedicine, Loxo Oncology/Lilly, and Nurix Therapeutics, unrelated to the current manuscript. D.B.S. has consulted for/received honoraria from Rain, Pfizer, Fog Pharma, Paige. AI, BridgeBio, Scorpion Therapeutics, FORE Therapeutics, Function Oncology, Pyramid, and Elsie Biotechnologies Inc. E.L.D. discloses unpaid editorial support from Pfizer Inc and serves on an advisory board for Day One Therapeutics and Springworks Therapeutics, both outside the submitted work. Alpelisib was kindly provided to the patient via the Managed Access Program at Novartis. The remaining authors declare no competing financial interests.
Figures



References
-
- Goyal G, Tazi A, Go RS, et al. International expert consensus recommendations for the diagnosis and treatment of Langerhans cell histiocytosis in adults. Blood. 2022;139(17):2601–2621. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

