Comparisons of Non-Oral Immune-Related Adverse Events Among Patients With Cancer With Different Oral Toxicity Profiles
- PMID: 37874927
- PMCID: PMC10911904
- DOI: 10.1093/oncolo/oyad279
Comparisons of Non-Oral Immune-Related Adverse Events Among Patients With Cancer With Different Oral Toxicity Profiles
Erratum in
-
Correction to: Comparisons of Nonoral Immune-Related Adverse Events Among Patients With Cancer With Different Oral Toxicity Profiles.Oncologist. 2024 Jan 5;29(1):e167-e168. doi: 10.1093/oncolo/oyad318. Oncologist. 2024. PMID: 38019641 Free PMC article. No abstract available.
Abstract
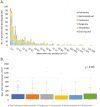
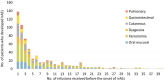
Objectives: Immune-related adverse events (irAEs) are common. Oral irAEs tend to cluster in patients who experience concurrent toxicities. We aimed to characterize the frequency and trajectory of non-oral irAEs in patients who developed oral irAEs, assess their relationship with non-oral irAEs, and compare those characteristics with patients without oral irAEs.
Methods: A retrospective chart review was conducted to identify patients who started ICIT between December 11, 2011, and September 15, 2019 (n = 4683) in the Mass General Brigham Registered Patient Data Registry. Demographic information, cancer diagnosis, ICIT regimen, treatment duration, and time and number of infusions to irAE onset were recorded. Non-oral irAEs were categorized into 13 groups. Patients with melanoma, pulmonary cancer, or head and neck cancer who had oral irAEs were then matched with those without oral irAEs to compare the prevalence of concomitant non-oral irAEs.
Results: Three hundred and fourteen patients with oral irAEs with a mean age of 65.9 ± 12.6 years (43.3% females) were included. Patients with multiple oral irAEs were more likely to have non-oral irAEs (OR: 2.7, 95% CI, 1.3-3.5), including cutaneous (OR: 1.7, 95% CI, 1.1-3.0), rheumatological (OR: 2.2, 95% CI, 1.1-4.2), thyroid (OR: 2.4, 95% CI, 1.2-4.9), and neurological irAEs (OR: 2.5, 95% CI, 1.0-6.3). Compared to matched patients with non-oral irAEs, patients with oral irAEs were more likely to have cutaneous (OR: 1.7, 95% CI, 1.0-2.8) and thyroid (OR: 2.86, 95% CI, 1.1-7.5) irAEs. The development of oral and non-oral irAEs is often coincidental.
Conclusion: Patients who have non-oral irAEs should be monitored for development of oral irAEs for prompt management.
Keywords: dysgeusia; head and neck cancer; immune checkpoint inhibitor therapy; immune-related adverse events; lung neoplasms; melanoma; oral mucositis; xerostomia.
© The Author(s) 2023. Published by Oxford University Press.
Conflict of interest statement
Robert I. Haddad: consultant/advisory role for Celgene, Merck, Eisai, Bristol-Myers Squibb, AstraZeneca, Pfizer, Loxo, Genentech, Immunomic Therapeutics, GSK, Gilead Sciences, Vaccinex, EMD Serono, BioNTech AG, Achilles Therapeutics, Bayer, Coherus Biosciences, Boehringer Ingelheim, MIRATI; research grant (to institution) from Boehringer Ingelheim, Merck, Bristol-Myers Squibb, Celgene, AstraZeneca, Genentech, Pfizer, Kura; royalties from UpToDate; data safety monitoring board for Nanobiotix, ISA Pharmaceuticals. Stephen T. Sonis: personal fees from Biomodels, LLC and Primary Endpoint Solutions, LLC. Alessandro Villa: research grant (to institution) from PCCA; royalties from UpToDate. The other authors indicated no financial relationships.
Figures