Fused Tetrahydroquinolines Are Interfering with Your Assay
- PMID: 37874947
- PMCID: PMC10641811
- DOI: 10.1021/acs.jmedchem.3c01277
Fused Tetrahydroquinolines Are Interfering with Your Assay
Abstract
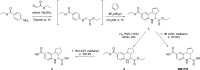
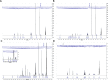
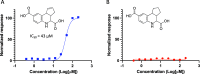
Tricyclic tetrahydroquinolines (THQs) have been repeatedly reported as hits across a diverse range of high-throughput screening (HTS) campaigns. The activities of these compounds, however, are likely due to reactive byproducts that interfere with the assay. As a lesser studied class of pan-assay interference compounds, the mechanism by which fused THQs react with protein targets remains largely unknown. During HTS follow-up, we characterized the behavior and stability of several fused tricyclic THQs. We synthesized key analogues to pinpoint the cyclopentene ring double bond as a source of reactivity of fused THQs. We found that these compounds degrade in solution under standard laboratory conditions in days. Importantly, these observations make it likely that fused THQs, which are ubiquitously found within small molecule screening libraries, are unlikely the intact parent compounds. We urge deprioritization of tricylic THQ hits in HTS follow-up and caution against the investment of resources to follow-up on these problematic compounds.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Chemical Information
Research Materials

