Universal Pairwise Interatomic van der Waals Potentials Based on Quantum Drude Oscillators
- PMID: 37875419
- PMCID: PMC10653113
- DOI: 10.1021/acs.jctc.3c00797
Universal Pairwise Interatomic van der Waals Potentials Based on Quantum Drude Oscillators
Abstract
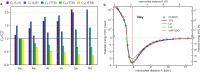
Repulsive short-range and attractive long-range van der Waals (vdW) forces play an appreciable role in the behavior of extended molecular systems. When using empirical force fields, the most popular computational methods applied to such systems, vdW forces are typically described by Lennard-Jones-like potentials, which unfortunately have a limited predictive power. Here, we present a universal parameterization of a quantum-mechanical vdW potential, which requires only two free-atom properties─the static dipole polarizability α1 and the dipole-dipole C6 dispersion coefficient. This is achieved by deriving the functional form of the potential from the quantum Drude oscillator (QDO) model, employing scaling laws for the equilibrium distance and the binding energy, and applying the microscopic law of corresponding states. The vdW-QDO potential is shown to be accurate for vdW binding energy curves, as demonstrated by comparing to the ab initio binding curves of 21 noble-gas dimers. The functional form of the vdW-QDO potential has the correct asymptotic behavior at both zero and infinite distances. In addition, it is shown that the damped vdW-QDO potential can accurately describe vdW interactions in dimers consisting of group II elements. Finally, we demonstrate the applicability of the atom-in-molecule vdW-QDO model for predicting accurate dispersion energies for molecular systems. The present work makes an important step toward constructing universal vdW potentials, which could benefit (bio)molecular computational studies.
Conflict of interest statement
The authors declare no competing financial interest.
Figures






References
-
- Stone A.The Theory of Intermolecular Forces; Oxford University Press, 2013.
-
- Kaplan I. G.Intermolecular Interactions: Physical Picture, Computational Methods and Model Potentials; John Wiley & Sons, 2006.
-
- Israelachvili J. N.Intermolecular and Surface Forces; Academic Press, 2011.
LinkOut - more resources
Full Text Sources

