Efficacy and safety of zuranolone co-initiated with an antidepressant in adults with major depressive disorder: results from the phase 3 CORAL study
- PMID: 37875578
- PMCID: PMC10724299
- DOI: 10.1038/s41386-023-01751-9
Efficacy and safety of zuranolone co-initiated with an antidepressant in adults with major depressive disorder: results from the phase 3 CORAL study
Abstract
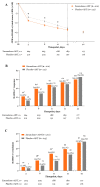
Major depressive disorder (MDD) is a mental health disorder that can cause disability and functional impairment that standard-of-care (SOC) antidepressant therapies (ADTs) can take weeks to treat. Zuranolone is a neuroactive steroid and positive allosteric modulator of synaptic and extrasynaptic γ-aminobutyric acid (GABA) type A receptors approved as an oral, once-daily, 14-day treatment course in adults with postpartum depression and under investigation in adults with MDD. The phase 3 CORAL Study (NCT04476030) evaluated the efficacy and safety of zuranolone 50 mg co-initiated with SOC ADT (zuranolone+ADT) vs placebo co-initiated with SOC ADT (placebo+ADT) in adults with MDD. Patients were randomized 1:1 to once-daily, blinded zuranolone+ADT or placebo+ADT for 14 days, then continued open-label SOC ADT for 28 more days. The primary endpoint was change from baseline (CFB) in the 17-item Hamilton Rating Scale for Depression (HAMD-17) total score at Day 3. Among 425 patients in the full analysis set, CFB in HAMD-17 total score at Day 3 was significantly improved with zuranolone+ADT vs placebo+ADT (least squares mean [standard error], -8.9 [0.39] vs -7.0 [0.38]; p = 0.0004). The majority of patients receiving zuranolone+ADT that experienced treatment-emergent adverse events (TEAEs) reported mild or moderate events. The most common TEAEs present in ≥10% of patients in either zuranolone+ADT or placebo+ADT groups were somnolence, dizziness, headache, and nausea. These results demonstrate that zuranolone+ADT provided more rapid improvement in depressive symptoms compared with placebo+ADT in patients with MDD, with a safety profile consistent with previous studies. Clinical trial registration: ClinicalTrials.gov identifier: NCT04476030.
© 2023. The Author(s).
Conflict of interest statement
SVP has received honoraria or research funds from Aifred; Assurex; Janssen; Mensante; Sage Therapeutics, Inc.; and Takeda. STA has received research support from COMPASS Pathways and Neuronetics; has served as a consultant to Genomind, Inc.; Janssen; LivaNova PLC; Neuronetics; and Sage Therapeutics, Inc.; and has served on speaker boards for Janssen and Sunovion Pharmaceuticals, Inc. SJM has served as a consultant to Allergan; Alkermes; Almatica Pharma; Axsome Therapeutics; BioXcel Therapeutics; Boehringer-Ingelheim; Clexio Biosciences; COMPASS Pathways; Eleusis; EMA Wellness; Engrail Therapeutics; Greenwich Biosciences; Intra-Cellular Therapies; Janssen; Levo Therapeutics; Perception Neurosciences; Praxis Precision Medicines; Neumora; Neurocrine; Relmada Therapeutics; Sage Therapeutics, Inc.; Seelos Therapeutics; Signant Health; and Sunovion and has received research support from Biohaven Pharmaceuticals; Boehringer-Ingelheim; Janssen; Merck; Sage Therapeutics, Inc.; and VistaGen Therapeutics. GA has received research support from Accera, Allergan, Axovant, Eisai, Genentech, Intra-Cellular Therapies, Janssen, Lundbeck, Neurim, Neurotrope, Novartis, Otsuka, Roche, Suven, and TransTech, and has served on the speakers bureau or as a consultant for Acadia, Alkermes, Allergan, Avanir, Janssen, Lundbeck, Merck, Nestle, Otsuka, Sunovion, Takeda, and Vanda. CD has received grant support from Biolite, Janssen, Neuronetics, and St. Jude, and has served on the advisory board of Alkermes. SK was an employee of Sage Therapeutics, Inc., at the time this trial was conducted and may hold stock in Sage Therapeutics, Inc., but is currently an employee of EmbarkNeuro. RL, AB, JJu, JJo, TV, and JD are employees of Sage Therapeutics, Inc., and may hold stock and/or stock options. MK, FF, and BL are employees of Biogen, Inc., and may hold stock.
Figures


References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

