Tau-targeting therapies for Alzheimer disease: current status and future directions
- PMID: 37875627
- PMCID: PMC10965012
- DOI: 10.1038/s41582-023-00883-2
Tau-targeting therapies for Alzheimer disease: current status and future directions
Abstract
Alzheimer disease (AD) is the most common cause of dementia in older individuals. AD is characterized pathologically by amyloid-β (Aβ) plaques and tau neurofibrillary tangles in the brain, with associated loss of synapses and neurons, which eventually results in dementia. Many of the early attempts to develop treatments for AD focused on Aβ, but a lack of efficacy of these treatments in terms of slowing disease progression led to a change of strategy towards targeting of tau pathology. Given that tau shows a stronger correlation with symptom severity than does Aβ, targeting of tau is more likely to be efficacious once cognitive decline begins. Anti-tau therapies initially focused on post-translational modifications, inhibition of tau aggregation and stabilization of microtubules. However, trials of many potential drugs were discontinued because of toxicity and/or lack of efficacy. Currently, the majority of tau-targeting agents in clinical trials are immunotherapies. In this Review, we provide an update on the results from the initial immunotherapy trials and an overview of new therapeutic candidates that are in clinical development, as well as considering future directions for tau-targeting therapies.
© 2023. Springer Nature Limited.
Conflict of interest statement
Competing interests
E. M. S. in an inventor on various patents related to the topic of this review that are assigned to New York University. Some of the patents on tau immunotherapies are licensed to H. Lundbeck. The other authors declare no competing interests.
Figures
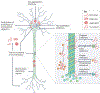


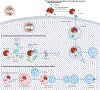
References
-
- Alzheimer’s Association. Alzheimer’s disease Facts and Figures 2022. Alzheimer Dement 18, 700–789 (2022). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

