Immunomodulatory effect of probiotic exopolysaccharides in a porcine in vitro co-culture model mimicking the intestinal environment on ETEC infection
- PMID: 37875712
- PMCID: PMC10998797
- DOI: 10.1007/s11259-023-10237-4
Immunomodulatory effect of probiotic exopolysaccharides in a porcine in vitro co-culture model mimicking the intestinal environment on ETEC infection
Abstract
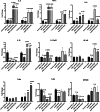
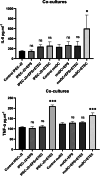
The aim of this study was to evaluate the immunomodulatory effect of EPS-L26 isolated from the probiotic strain Lactobacillus (Limosilactobacillus) reuteri L26 Biocenol™, in a model of infection with an enterotoxigenic E. coli (ETEC) by establishing monocultures consisting of the IPEC-J2 cell line or monocyte-derived dendritic cells (moDCs) and creating a 3D model of cell co-cultures established with IPEC-J2 cells and moDCs. The immunomodulatory and immunoprotective potential of used EPS-L26 was confirmed in monocultures in an experimental group of pretreated cells, where our study showed that pretreatment of cells with EPS-L26 and subsequent exposure to infection resulted in significantly down-regulated mRNA levels of genes encoding inflammatory cytokines compared to ETEC challenge in single cell cultures (in IPEC-J2, decreased mRNA levels for TNF-α, IL-6, IL-1β, IL-12p35; in moDCs, decreased mRNA levels for IL-1β). Similar to monocultures, we also demonstrated the immunostimulatory potential of the ETEC strain in the co-culture model on directly treated IPEC-J2 cells cultivated on insert chambers (apical compartment) and also on indirectly treated moDCs cultivated in the lower chamber (basolateral compartment), however in the co-culture model the expression of inflammatory cytokines was attenuated at the mRNA level compared to monocultures. Pretreatment of the cells on the insert chambers pointed to the immunoprotective properties of EPS-L26, manifested by decreased mRNA levels in both cell lines compared to ETEC challenge (in IPEC-J2 decreased mRNA levels for IL-12p35; in moDCs decreased mRNA levels for IL-1β, IL-6). Our results suggest intercellular communication via humoral signals derived from IPEC-J2 cells by influencing the gene expression of indirectly treated moDC cells located in the basolateral compartment.
Keywords: Co-culture; Cytokines; EPS; ETEC; IPEC-J2; moDCs; qPCR.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
The authors have no pertinent financial or non-financial conflicts of interest to declare.
Figures








References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

