Complement-membrane regulatory proteins are absent from the nodes of Ranvier in the peripheral nervous system
- PMID: 37875972
- PMCID: PMC10594684
- DOI: 10.1186/s12974-023-02920-9
Complement-membrane regulatory proteins are absent from the nodes of Ranvier in the peripheral nervous system
Abstract
Background: Homozygous CD59-deficient patients manifest with recurrent peripheral neuropathy resembling Guillain-Barré syndrome (GBS), hemolytic anemia and recurrent strokes. Variable mutations in CD59 leading to loss of function have been described and, overall, 17/18 of patients with any mutation presented with recurrent GBS. Here we determine the localization and possible role of membrane-bound complement regulators, including CD59, in the peripheral nervous systems (PNS) of mice and humans.
Methods: We examined the localization of membrane-bound complement regulators in the peripheral nerves of healthy humans and a CD59-deficient patient, as well as in wild-type (WT) and CD59a-deficient mice. Cross sections of teased sciatic nerves and myelinating dorsal root ganglia (DRG) neuron/Schwann cell cultures were examined by confocal and electron microscopy.
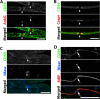
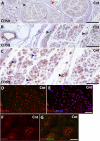
Results: We demonstrate that CD59a-deficient mice display normal peripheral nerve morphology but develop myelin abnormalities in older age. They normally express myelin protein zero (P0), ankyrin G (AnkG), Caspr, dystroglycan, and neurofascin. Immunolabeling of WT nerves using antibodies to CD59 and myelin basic protein (MBP), P0, and AnkG revealed that CD59 was localized along the internode but was absent from the nodes of Ranvier. CD59 was also detected in blood vessels within the nerve. Finally, we show that the nodes of Ranvier lack other complement-membrane regulatory proteins, including CD46, CD55, CD35, and CR1-related gene-y (Crry), rendering this area highly exposed to complement attack.
Conclusion: The Nodes of Ranvier lack CD59 and are hence not protected from complement terminal attack. The myelin unit in human PNS is protected by CD59 and CD55, but not by CD46 or CD35. This renders the nodes and myelin in the PNS vulnerable to complement attack and demyelination in autoinflammatory Guillain-Barré syndrome, as seen in CD59 deficiency.
Keywords: CD59; Complement; Guillain-Barré syndrome; Myelin; Nodes of Ranvier; Nodopathies; Peripheral nervous system.
© 2023. BioMed Central Ltd., part of Springer Nature.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Yuki N, Hartung HP. Guillain-Barre syndrome. N Engl J Med. 2012;366:2294–2304. - PubMed
-
- Mevorach D, Reiner I, Grau A, Ilan U, Berkun Y, Ta-Shma A, et al. Therapy with eculizumab for patients with CD59 p.Cys89Tyr mutation. Ann Neurol. 2016;80:708–717. - PubMed
-
- Tabib A, Karbian N, Mevorach D. Demyelination, strokes, and eculizumab: lessons from the congenital CD59 gene mutations. Mol Immunol. 2017;89:69–72. - PubMed
-
- Ardicli D, Taskiran EZ, Kosukcu C, Temucin C, Oguz KK, Haliloglu G, et al. Neonatal-onset recurrent Guillain-Barre syndrome-like disease: clues for inherited CD59 deficiency. Neuropediatrics. 2017;48:477–481. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

