Plasticity in the cell division processes of obligate intracellular bacteria
- PMID: 37876871
- PMCID: PMC10591338
- DOI: 10.3389/fcimb.2023.1205488
Plasticity in the cell division processes of obligate intracellular bacteria
Abstract
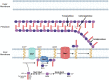
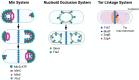
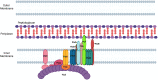
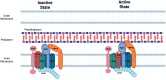
Most bacteria divide through a highly conserved process called binary fission, in which there is symmetric growth of daughter cells and the synthesis of peptidoglycan at the mid-cell to enable cytokinesis. During this process, the parental cell replicates its chromosomal DNA and segregates replicated chromosomes into the daughter cells. The mechanisms that regulate binary fission have been extensively studied in several model organisms, including Eschericia coli, Bacillus subtilis, and Caulobacter crescentus. These analyses have revealed that a multi-protein complex called the divisome forms at the mid-cell to enable peptidoglycan synthesis and septation during division. In addition, rod-shaped bacteria form a multi-protein complex called the elongasome that drives sidewall peptidoglycan synthesis necessary for the maintenance of rod shape and the lengthening of the cell prior to division. In adapting to their intracellular niche, the obligate intracellular bacteria discussed here have eliminated one to several of the divisome gene products essential for binary fission in E. coli. In addition, genes that encode components of the elongasome, which were mostly lost as rod-shaped bacteria evolved into coccoid organisms, have been retained during the reductive evolutionary process that some coccoid obligate intracellular bacteria have undergone. Although the precise molecular mechanisms that regulate the division of obligate intracellular bacteria remain undefined, the studies summarized here indicate that obligate intracellular bacteria exhibit remarkable plasticity in their cell division processes.
Keywords: cell division; divisome; elongasome; obligate intracellular bacteria; peptidoglycan.
Copyright © 2023 Harpring and Cox.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
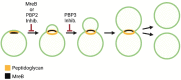
Similar articles
-
FtsK is critical for the assembly of the unique divisome complex of the FtsZ-less Chlamydia trachomatis.Elife. 2025 Apr 7;13:RP104199. doi: 10.7554/eLife.104199. Elife. 2025. PMID: 40193186 Free PMC article.
-
Elongation at Midcell in Preparation of Cell Division Requires FtsZ, but Not MreB nor PBP2 in Caulobacter crescentus.Front Microbiol. 2021 Aug 27;12:732031. doi: 10.3389/fmicb.2021.732031. eCollection 2021. Front Microbiol. 2021. PMID: 34512611 Free PMC article.
-
Morphogenesis of rod-shaped sacculi.FEMS Microbiol Rev. 2008 Mar;32(2):321-44. doi: 10.1111/j.1574-6976.2007.00090.x. FEMS Microbiol Rev. 2008. PMID: 18291013 Review.
-
Division without Binary Fission: Cell Division in the FtsZ-Less Chlamydia.J Bacteriol. 2020 Aug 10;202(17):e00252-20. doi: 10.1128/JB.00252-20. Print 2020 Aug 10. J Bacteriol. 2020. PMID: 32540934 Free PMC article. Review.
-
From models to pathogens: how much have we learned about Streptococcus pneumoniae cell division?Environ Microbiol. 2013 Dec;15(12):3133-57. doi: 10.1111/1462-2920.12189. Epub 2013 Jul 15. Environ Microbiol. 2013. PMID: 23848140 Review.
Cited by
-
FtsK is critical for the assembly of the unique divisome complex of the FtsZ-less Chlamydia trachomatis.Elife. 2025 Apr 7;13:RP104199. doi: 10.7554/eLife.104199. Elife. 2025. PMID: 40193186 Free PMC article.
-
Frequent and asymmetric cell division in endosymbiotic bacteria of cockroaches.Appl Environ Microbiol. 2024 Oct 23;90(10):e0146624. doi: 10.1128/aem.01466-24. Epub 2024 Sep 18. Appl Environ Microbiol. 2024. PMID: 39291985 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

