Early-stage mapping of macromolecular content in APPNL-F mouse model of Alzheimer's disease using nuclear Overhauser effect MRI
- PMID: 37876875
- PMCID: PMC10590923
- DOI: 10.3389/fnagi.2023.1266859
Early-stage mapping of macromolecular content in APPNL-F mouse model of Alzheimer's disease using nuclear Overhauser effect MRI
Abstract
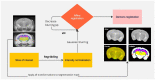
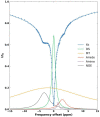
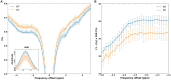
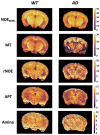
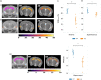
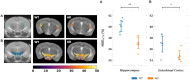
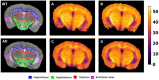
Non-invasive methods of detecting early-stage Alzheimer's disease (AD) can provide valuable insight into disease pathology, improving the diagnosis and treatment of AD. Nuclear Overhauser enhancement (NOE) MRI is a technique that provides image contrast sensitive to lipid and protein content in the brain. These macromolecules have been shown to be altered in Alzheimer's pathology, with early disruptions in cell membrane integrity and signaling pathways leading to the buildup of amyloid-beta plaques and neurofibrillary tangles. We used template-based analyzes of NOE MRI data and the characteristic Z-spectrum, with parameters optimized for increase specificity to NOE, to detect changes in lipids and proteins in an AD mouse model that recapitulates features of human AD. We find changes in NOE contrast in the hippocampus, hypothalamus, entorhinal cortex, and fimbria, with these changes likely attributed to disruptions in the phospholipid bilayer of cell membranes in both gray and white matter regions. This study suggests that NOE MRI may be a useful tool for monitoring early-stage changes in lipid-mediated metabolism in AD and other disorders with high spatial resolution.
Keywords: Alzheimer’s disease; CEST; NOE; lipid dyshomeostasis; multipool fitting.
Copyright © 2023 Swain, Soni, Wilson, Juul, Benyard, Haris, Kumar, Nanga, Detre, Lee and Reddy.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Bao Y. W., Chau A. C. M., Chiu P. K., Shea Y. F., Kwan J. S. K., Chan F. H. W., et al. (2021). Heterogeneity of amyloid binding in cognitively impaired patients consecutively recruited from a memory clinic: evaluating the utility of quantitative 18F-Flutemetamol PET-CT in discrimination of mild cognitive impairment from Alzheimer's disease and other dementias. J. Alzheimers Dis. 79, 819–832. doi: 10.3233/JAD-200890, PMID: - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

