The fungal-derived compound AM3 modulates pro-inflammatory cytokine production and skews the differentiation of human monocytes
- PMID: 37876931
- PMCID: PMC10591448
- DOI: 10.3389/fimmu.2023.1165683
The fungal-derived compound AM3 modulates pro-inflammatory cytokine production and skews the differentiation of human monocytes
Abstract
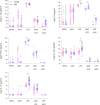
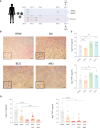
The proper functioning of the immune system depends on an appropriate balance between pro-inflammation and anti-inflammation. When the balance is disrupted and the system is excessively biased towards inflammation, immune responses cannot return within the normal range, which favors the onset of diseases of autoimmune or inflammatory nature. In this scenario, it is fundamental to find new compounds that can help restore this balance and contribute to the normal functioning of the immune system in humans. Here, we show the properties of a fungal compound with a strong safety profile in humans, AM3, as an immunomodulatory molecule to decrease excessive cytokine production in human cells. Our results present that AM3 treatment of human peripheral blood mononuclear cells and monocytes decreased their pro-inflammatory cytokine secretion following the challenge with bacterial lipopolysaccharide. Additionally, AM3 skewed the differentiation profile of human monocytes to macrophages towards a non-inflammatory phenotype without inducing tolerance, meaning these cells kept their capacity to respond to different stimuli. These effects were similar in young and elderly individuals. Thus, the fungal compound, AM3 may help reduce excessive immune activation in inflammatory conditions and keep the immune responses within a normal homeostatic range, regardless of the age of the individual.
Keywords: AM3; cytokines; disease; homeostasis; immune response; inflammation; macrophages; monocytes.
Copyright © 2023 Geckin, Kilic, Debisarun, Föhse, Rodríguez-Luna, Fernández-González, Sánchez and Domínguez-Andrés.
Conflict of interest statement
AR-L and AL were full-time employed by Cantabria Labs. PF-G is a scientific adviser at Cantabria Labs. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

