Neurologic Effects of SARS-CoV-2 Transmitted among Dogs
- PMID: 37877548
- PMCID: PMC10617347
- DOI: 10.3201/eid2911.230804
Neurologic Effects of SARS-CoV-2 Transmitted among Dogs
Abstract
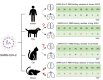
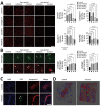
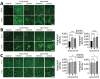
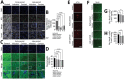
SARS-CoV-2 induces illness and death in humans by causing systemic infections. Evidence suggests that SARS-CoV-2 can induce brain pathology in humans and other hosts. In this study, we used a canine transmission model to examine histopathologic changes in the brains of dogs infected with SARS-CoV-2. We observed substantial brain pathology in SARS-CoV-2-infected dogs, particularly involving blood-brain barrier damage resembling small vessel disease, including changes in tight junction proteins, reduced laminin levels, and decreased pericyte coverage. Furthermore, we detected phosphorylated tau, a marker of neurodegenerative disease, indicating a potential link between SARS-CoV-2-associated small vessel disease and neurodegeneration. Our findings of degenerative changes in the dog brain during SARS-CoV-2 infection emphasize the potential for transmission to other hosts and induction of similar signs and symptoms. The dynamic brain changes in dogs highlight that even asymptomatic individuals infected with SARS-CoV-2 may develop neuropathologic changes in the brain.
Keywords: 2019 novel coronavirus disease; COVID-19; SARS-CoV-2; South Korea; blood–brain barrier; coronavirus disease; dogs; infection transmission; neurodegenerative diseases; neurological model; respiratory infections; severe acute respiratory syndrome coronavirus 2; viruses; zoonoses.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

