Sigh Ventilation in Patients With Trauma: The SiVent Randomized Clinical Trial
- PMID: 37877609
- PMCID: PMC10600720
- DOI: 10.1001/jama.2023.21739
Sigh Ventilation in Patients With Trauma: The SiVent Randomized Clinical Trial
Abstract
Importance: Among patients receiving mechanical ventilation, tidal volumes with each breath are often constant or similar. This may lead to ventilator-induced lung injury by altering or depleting surfactant. The role of sigh breaths in reducing ventilator-induced lung injury among trauma patients at risk of poor outcomes is unknown.
Objective: To determine whether adding sigh breaths improves clinical outcomes.
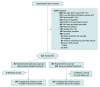
Design, setting, and participants: A pragmatic, randomized trial of sigh breaths plus usual care conducted from 2016 to 2022 with 28-day follow-up in 15 academic trauma centers in the US. Inclusion criteria were age older than 18 years, mechanical ventilation because of trauma for less than 24 hours, 1 or more of 5 risk factors for developing acute respiratory distress syndrome, expected duration of ventilation longer than 24 hours, and predicted survival longer than 48 hours.
Interventions: Sigh volumes producing plateau pressures of 35 cm H2O (or 40 cm H2O for inpatients with body mass indexes >35) delivered once every 6 minutes. Usual care was defined as the patient's physician(s) treating the patient as they wished.
Main outcomes and measures: The primary outcome was ventilator-free days. Prespecified secondary outcomes included all-cause 28-day mortality.
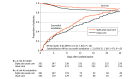
Results: Of 5753 patients screened, 524 were enrolled (mean [SD] age, 43.9 [19.2] years; 394 [75.2%] were male). The median ventilator-free days was 18.4 (IQR, 7.0-25.2) in patients randomized to sighs and 16.1 (IQR, 1.1-24.4) in those receiving usual care alone (P = .08). The unadjusted mean difference in ventilator-free days between groups was 1.9 days (95% CI, 0.1 to 3.6) and the prespecified adjusted mean difference was 1.4 days (95% CI, -0.2 to 3.0). For the prespecified secondary outcome, patients randomized to sighs had 28-day mortality of 11.6% (30/259) vs 17.6% (46/261) in those receiving usual care (P = .05). No differences were observed in nonfatal adverse events comparing patients with sighs (80/259 [30.9%]) vs those without (80/261 [30.7%]).
Conclusions and relevance: In a pragmatic, randomized trial among trauma patients receiving mechanical ventilation with risk factors for developing acute respiratory distress syndrome, the addition of sigh breaths did not significantly increase ventilator-free days. Prespecified secondary outcome data suggest that sighs are well-tolerated and may improve clinical outcomes.
Trial registration: ClinicalTrials.gov Identifier: NCT02582957.
Conflict of interest statement
Figures
Comment in
-
Sigh Breaths for Trauma Patients Receiving Mechanical Ventilation: Take a Deep Breath.JAMA. 2023 Nov 28;330(20):1956-1957. doi: 10.1001/jama.2023.21744. JAMA. 2023. PMID: 37877626 No abstract available.
-
Sigh Breaths in Patients With Trauma Receiving Mechanical Ventilation.JAMA. 2024 Mar 12;331(10):887-888. doi: 10.1001/jama.2024.0117. JAMA. 2024. PMID: 38470389 No abstract available.
References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical

