Work loss and activity impairment due to extended nausea and vomiting in patients with breast cancer receiving CINV prophylaxis
- PMID: 37878086
- PMCID: PMC10600031
- DOI: 10.1007/s00520-023-08119-1
Work loss and activity impairment due to extended nausea and vomiting in patients with breast cancer receiving CINV prophylaxis
Abstract
Purpose: Chemotherapy-induced nausea and vomiting (CINV)'s impact on work loss remains poorly described. We evaluated associations between the duration of CINV episodes, CINV-related work loss (CINV-WL), and CINV-related activity impairment (CINV-AI) in patients with breast cancer receiving highly emetogenic chemotherapy.
Methods: We analyzed data from a prospective CINV prophylaxis trial of netupitant/palonestron and dexamethasone for patients receiving an anthracycline and cyclophosphamide (AC) for breast cancer (NCT0340371). Over the observed CINV duration (0-5 days), we analyzed patient-reported CINV-WL and CINV-AI for the first two chemotherapy cycles. We categorized patients as having either extended (≥ 3 days) or short (1-2 days) CINV duration and quantified its impact on work using the Work Productivity and Activity Impairment Questionnaire (WPAI).
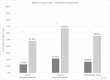
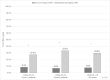
Results: Overall, we captured data for 792 cycles in 402 women, including 136 (33.8%) employed patients with 35.3% reporting CINV. Of those with CINV, patients reported CINV-WL in 26 cycles and CINV-AI in 142 cycles. Of those with CINV, 55.3% of extended CINV cycles experienced CINV-WL compared to 16.7% of short CINV cycles (p < 0.001). The relative risk of CINV-WL between extended and short CINV was 3.32 (p < 0.01) for employed patients. The mean difference in CINV-AI scores (higher = worse) between extended and short duration CINV was 5.0 vs. 3.0 (p < 0.001).
Conclusion: Extended (≥ 3 days) CINV was associated with more than triple the risk of CINV-WL and higher CINV-AI compared with short CINV.
Keywords: Antiemetic; Breast cancer; CINV; Chemotherapy; Work loss.
© 2023. The Author(s).
Conflict of interest statement
Mr. Binder, Mr. Bailey, and Mr. Turini were employees of Helsinn at the time the study was conducted. Dr. Roeland reports a consulting/advisory role for Napo, AIM Specialty Health, Oragenics, BASF, Immuneering, Vector Oncology, Asahi Kasei, Heron, Pfizer/EMD Serono, and Mitobridge. Dr. LeBlanc reports personal fees for consulting or advisory boards from AbbVie, Agios/Servier, Astellas, BlueNote, BMS/Celgene, Flatiron, Genentech, GSK, and Pfizer; royalties from UpToDate; speakers bureau fees from Agios/Servier, AbbVie, and BMS/Celgene; grants and/or research contracts from the American Cancer Society, AstraZeneca, BMS, Jazz Pharmaceuticals, the NINR / NIH, and Seattle Genetics. Dr. Wickham reports honoraria from Insys Therapeutics, and scientific advisory board fees from Helsinn. Mr. Potluri, Mr. Papademetriou, and Ms. Liu were employees of Putnam PHMR at the time of the study and received payment for conducting the analyses. Dr. Schwartzberg reports consulting fees from Pfizer, Myriad, Genentech, Odonate, Amgen, Spectrum, Napo, Lilly, BMS, Helsinn, Bayer, BeyondSpring, AstraZeneca, Seagen, Sanofi, Coherus; speakers bureau fees from Coherus, Puma, Merck, Seagen, and Pfizer. Drs. Navari, Ruddy, Kloth, and Ms. Clark-Snow report no conflicts of interest.
Figures
References
-
- Osoba D, Zee B, Warr D, et al. Effect of postchemotherapy nausea and vomiting on health-related quality of life. the quality of life and symptom control committees of the National Cancer Institute of Canada Clinical Trials Group. Support Care Cancer. 1997;5:307–313. doi: 10.1007/S005200050078. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical