Effectiveness of Surgeon-Performed Paravertebral Block Analgesia for Minimally Invasive Thoracic Surgery: A Randomized Clinical Trial
- PMID: 37878299
- PMCID: PMC10600725
- DOI: 10.1001/jamasurg.2023.5228
Effectiveness of Surgeon-Performed Paravertebral Block Analgesia for Minimally Invasive Thoracic Surgery: A Randomized Clinical Trial
Abstract
Importance: In minimally invasive thoracic surgery, paravertebral block (PVB) using ultrasound (US)-guided technique is an efficient postoperative analgesia. However, it is an operator-dependent process depending on experience and local resources. Because pain-control failure is highly detrimental, surgeons may consider other locoregional analgesic options.
Objective: To demonstrate the noninferiority of PVB performed by surgeons under video-assisted thoracoscopic surgery (VATS), hereafter referred to as PVB-VATS, as the experimental group compared with PVB performed by anesthesiologists using US-guided technique (PVB-US) as the control group.
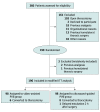
Design, setting, and participants: In this single-center, noninferiority, patient-blinded, randomized clinical trial conducted from September 8, 2020, to December 8, 2021, patients older than 18 years who were undergoing a scheduled minimally invasive thoracic surgery with lung resection including video-assisted or robotic approaches were included. Exclusion criteria included scheduled open surgery, any antalgic World Health Organization level greater than 2 before surgery, or a medical history of homolateral thoracic surgery. Patients were randomly assigned (1:1) to an intervention group after general anesthesia. They received single-injection PVB before the first incision was made in the control group (PVB-US) or after 1 incision was made under thoracoscopic vision in the experimental group (PVB-VATS).
Interventions: PVB-VATS or PVB-US.
Main outcomes and measures: The primary end point was mean 48-hour post-PVB opioid consumption considering a noninferiority range of less than 7.5 mg of opioid consumption between groups. Secondary outcomes included time of anesthesia, surgery, and operating room occupancy; 48-hour pain visual analog scale score at rest and while coughing; and 30-day postoperative complications.
Results: A total of 196 patients were randomly assigned to intervention groups: 98 in the PVB-VATS group (mean [SD] age, 64.6 [9.5] years; 53 female [54.1%]) and 98 in the PVB-US group (mean [SD] age, 65.8 [11.5] years; 62 male [63.3%]). The mean (SD) of 48-hour opioid consumption in the PVB-VATS group (33.9 [19.8] mg; 95% CI, 30.0-37.9 mg) was noninferior to that measured in the PVB-US group (28.5 [18.2] mg; 95% CI, 24.8-32.2 mg; difference: -5.4 mg; 95% CI, -∞ to -0.93; noninferiority Welsh test, P ≤ .001). Pain score at rest and while coughing after surgery, overall time, and postoperative complications did not differ between groups.
Conclusions and relevance: PVB placed by a surgeon during thoracoscopy was noninferior to PVB placed by an anesthesiologist using ultrasonography before incision in terms of opioid consumption during the first 48 hours.
Trial registration: ClinicalTrials.gov Identifier: NCT04579276.
Conflict of interest statement
Figures


Comment in
-
The Delineation of Another Standard for Postoperative Pain Management Following Thoracic Surgery.JAMA Surg. 2023 Dec 1;158(12):1263-1264. doi: 10.1001/jamasurg.2023.5229. JAMA Surg. 2023. PMID: 37878290 No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

