Metformin Cessation and Dementia Incidence
- PMID: 37878309
- PMCID: PMC10600586
- DOI: 10.1001/jamanetworkopen.2023.39723
Metformin Cessation and Dementia Incidence
Abstract
Importance: Prior studies suggested that metformin may be associated with reduced dementia incidence, but associations may be confounded by disease severity and prescribing trends. Cessation of metformin therapy in people with diabetes typically occurs due to signs of kidney dysfunction but sometimes is due to less serious adverse effects associated with metformin.
Objective: To investigate the association of terminating metformin treatment for reasons unrelated to kidney dysfunction with dementia incidence.
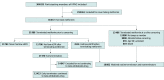
Design, setting, and participants: This cohort study was conducted at Kaiser Permanente Northern California, a large integrated health care delivery system, among a cohort of metformin users born prior to 1955 without history of diagnosed kidney disease at metformin initiation. Dementia follow-up began with the implementation of electronic health records in 1996 and continued to 2020. Data were analyzed from November 2021 through September 2023.
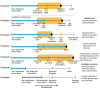
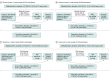
Exposures: A total of 12 220 early terminators, individuals who stopped metformin with normal estimated glomerular filtration rate (eGFR), were compared with routine metformin users, who had not yet terminated metformin treatment or had terminated (with or without restarting) after their first abnormal eGFR measurement. Early terminators were matched with routine users of the same age and gender who had diabetes for the same duration.
Main outcomes and measures: The outcome of interest was all-cause incident dementia. Follow-up for early terminators and their matched routine users was started at age of termination for the early terminator. Survival models adjusted for sociodemographic characteristics and comorbidities at the time of metformin termination (or matched age). Mediation models with HbA1c level and insulin usage 1 and 5 years after termination tested whether changes in blood glucose or insulin usage explained associations between early termination of metformin and dementia incidence.
Results: The final analytic sample consisted of 12 220 early terminators (5640 women [46.2%]; mean [SD] age at start of first metformin prescription, 59.4 [9.0] years) and 29 126 routine users (13 582 women [46.6%]; mean [SD] age at start of first metformin prescription, 61.1 [8.9] years). Early terminators had 1.21 times the hazard of dementia diagnosis compared with routine users (hazard ratio, 1.21; 95% CI, 1.12 to 1.30). In mediation analysis, contributions to this association by changes in HbA1c level or insulin use ranged from no contribution (0.00 years; 95% CI, -0.02 to 0.02 years) for insulin use at 5 years after termination to 0.07 years (95% CI, 0.02 to 0.13 years) for HbA1c level at 1 year after termination, suggesting that the association was largely independent of changes in HbA1c level and insulin usage.
Conclusions and relevance: In this study, terminating metformin treatment was associated with increased dementia incidence. This finding may have important implications for clinical treatment of adults with diabetes and provides additional evidence that metformin is associated with reduced dementia risk.
Conflict of interest statement
Figures
References
-
- Nathan DM, Buse JB, Davidson MB, et al. . Management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2006;29(8):1963-1972. doi:10.2337/dc06-9912 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

