Decreased hepatic thyroid hormone signaling in systemic and liver-specific but not brain-specific accelerated aging due to DNA repair deficiency in mice
- PMID: 37878415
- PMCID: PMC10762595
- DOI: 10.1530/ETJ-22-0231
Decreased hepatic thyroid hormone signaling in systemic and liver-specific but not brain-specific accelerated aging due to DNA repair deficiency in mice
Abstract
Background: Thyroid hormone signaling is essential for development, metabolism, and response to stress but declines during aging, the cause of which is unknown. DNA damage accumulating with time is a main cause of aging, driving many age-related diseases. Previous studies in normal and premature aging mice, due to defective DNA repair, indicated reduced hepatic thyroid hormone signaling accompanied by decreased type 1 deiodinase (DIO1) and increased DIO3 activities. We investigated whether aging-related changes in deiodinase activity are driven by systemic signals or represent cell- or organ-autonomous changes.
Methods: We quantified liver and plasma thyroid hormone concentrations, deiodinase activities and expression of T3-responsive genes in mice with a global, liver-specific and for comparison brain-specific inactivation of Xpg, one of the endonucleases critically involved in multiple DNA repair pathways.
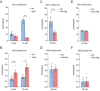
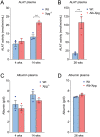
Results: Both in global and liver-specific Xpg knockout mice, hepatic DIO1 activity was decreased. Interestingly, hepatic DIO3 activity was increased in global, but not in liver-specific Xpg mutants. Selective Xpg deficiency and premature aging in the brain did not affect liver or systemic thyroid signaling. Concomitant with DIO1 inhibition, Xpg -/- and Alb-Xpg mice displayed reduced thyroid hormone-related gene expression changes, correlating with markers of liver damage and cellular senescence.
Conclusions: Our findings suggest that DIO1 activity during aging is predominantly modified in a tissue-autonomous manner driven by organ/cell-intrinsic accumulating DNA damage. The increase in hepatic DIO3 activity during aging largely depends on systemic signals, possibly reflecting the presence of circulating cells rather than activity in hepatocytes.
Keywords: DNA damage; aging; deiodinase; liver; nucleotide excision repair; progeria; thyroid hormone.
© the author(s) 2023
Conflict of interest statement
The authors declare that there is no conflict of interest that could prejudice the impartiality of the research reported. Edward Visser is on the editorial board of European Thyroid Journal. Edward Visser was not involved in the review or editorial process for this paper, on which he is listed as an author.
Figures





References
-
- Engels K Rakov H Hönes GS Brix K Köhrle J Zwanziger D Moeller LC & Führer D. Aging alters phenotypic traits of thyroid dysfunction in male mice with divergent effects on complex systems but preserved thyroid hormone action in target organs. Journals of Gerontology. Series A, Biological Sciences and Medical Sciences 2019741162–1169. (10.1093/gerona/glz040) - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

