The tumor microenvironment state associates with response to HPV therapeutic vaccination in patients with respiratory papillomatosis
- PMID: 37878675
- PMCID: PMC11279704
- DOI: 10.1126/scitranslmed.adj0740
The tumor microenvironment state associates with response to HPV therapeutic vaccination in patients with respiratory papillomatosis
Abstract
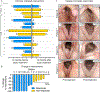
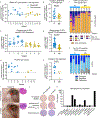
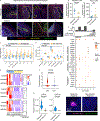
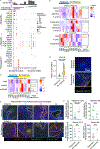
Recurrent respiratory papillomatosis (RRP) is a rare, debilitating neoplastic disorder caused by chronic infection with human papillomavirus (HPV) type 6 or 11 and characterized by growth of papillomas in the upper aerodigestive tract. There is no approved medical therapy, and patients require repeated debulking procedures to maintain voice and airway function. PRGN-2012 is a gorilla adenovirus immune-therapeutic capable of enhancing HPV 6/11-specific T cell immunity. This first-in-human, phase 1 study (NCT04724980) of adjuvant PRGN-2012 treatment in adult patients with severe, aggressive RRP demonstrates the overall safety and clinically meaningful benefit observed with PRGN-2012, with a 50% complete response rate in patients treated at the highest dose. Responders demonstrate greater expansion of peripheral HPV-specific T cells compared with nonresponders. Additional correlative studies identify an association between reduced baseline papilloma HPV gene expression, greater interferon responses and expression of CXCL9 and CXCL10, and greater papilloma T cell infiltration in responders. Conversely, nonresponders were characterized by greater HPV and CXCL8 gene expression, increased neutrophilic cell infiltration, and reduced T cell papilloma infiltration. These results suggest that papilloma HPV gene expression may regulate interferon signaling and chemokine expression profiles within the tumor microenvironment that cooperate to govern clinical response to therapeutic HPV vaccination in patients with respiratory papillomatosis.
Conflict of interest statement
Competing Interests
EW, AL, RS, HS and DEB are employees of Precigen, Inc. DEB has a patent related to this work titled ‘Human papillomavirus vaccines and uses of the same for HPV associated diseases’, patient number WO2022/115470. Otherwise the authors declare that no competing interests or consulting declarations exist.
Figures
References
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

