Count Me In: an inclusive approach towards patient recruitment for clinical research studies in the NHS
- PMID: 37879674
- PMCID: PMC10603415
- DOI: 10.1136/bmjment-2023-300774
Count Me In: an inclusive approach towards patient recruitment for clinical research studies in the NHS
Abstract
Background: Participation in clinical research is associated with better patient outcomes and higher staff retention and satisfaction rates. Nevertheless, patient recruitment to mental health studies is challenging due to a reliance on clinician or patient referrals (standard approach). To empower patients and make healthcare research more equitable, we explored a novel researcher-led approach, called 'Count Me In' (CMI).
Objective: To evaluate a 12-month implementation of CMI in a routine clinical setting.
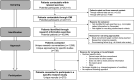
Methods: CMI was launched in August 2021 in a mental health National Health Service (NHS) Trust in England. Patients (aged 18+) learnt about CMI at their initial clinical appointment. Unless they opted out, they became contactable for research (via research informatics searches).
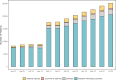
Findings: After 12 months, 368 patients opted out and 22 741 became contactable through CMI, including 2716 through the standard approach and 20 025 through electronic searches (637% increase). Of those identified via electronic searches, 738 were contacted about specific studies and 270 consented to participate. Five themes were identified based on patient and staff experiences of CMI: 'level of awareness and accessibility of CMI', 'perceptions of research and perceived engagement with CMI', 'inclusive research practice', 'engagement and incentives for research participation', and 'relationships between clinical and research settings'.
Conclusions: CMI (vs standard) led to a larger and diverse patient cohort and was favoured by patients and staff. Yet a shift in the NHS research culture is needed to ensure that this diversity translates to actual research participation.
Clinical implications: Through collaboration with other NHS Trusts and services, key funders (National Institute for Health and Care Research) and new national initiatives (Office for Life Sciences Mental Health Mission), CMI has the potential to address recruitment challenges through rapid patient recruitment into time-sensitive country-wide studies.
Keywords: Adult psychiatry.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. Published by BMJ.
Conflict of interest statement
Competing interests: AC has received research, educational and consultancy fees from INCiPiT (Italian Network for Paediatric Trials), CARIPLO Foundation, Lundbeck and Angelini Pharma.
Figures

References
-
- Johnson O. An evidence-based approach to conducting clinical trial feasibility assessments. Clinical Investigation 2015;5:491–9. 10.4155/cli.14.139 - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
