Pharmaceutical industry payments and delivery of non-recommended and low value cancer drugs: population based cohort study
- PMID: 37879723
- PMCID: PMC10599253
- DOI: 10.1136/bmj-2023-075512
Pharmaceutical industry payments and delivery of non-recommended and low value cancer drugs: population based cohort study
Abstract
Objective: To estimate the association between oncologists' receipt of payments from the pharmaceutical industry and delivery of non-recommended or low value interventions among their patients.
Design: Cohort study.
Setting: Fee-for-service Medicare claims.
Participants: Medicare beneficiaries with a diagnosis of incident cancer (new occurrence of a cancer diagnosis code in proximity to claims for cancer treatment, and no such diagnosis codes during a ≥1 year washout period) during 2014-19, who met additional requirements identifying them as at risk for one of four non-recommended or low value interventions: denosumab for castration sensitive prostate cancer, granulocyte colony stimulating factors (GCSF) for patients at low risk for neutropenic fever, nab-paclitaxel for cancers with no evidence of superiority over paclitaxel, and a branded drug in settings where a generic or biosimilar version was available.
Main outcome measures: Receipt of the non-recommended or low value drug for which the patient was at risk. The primary association of interest was the assigned oncologist's receipt of any general payments from the manufacturer of the corresponding non-recommended or low value drug (measured in Open Payments) within 365 days before the patient's index cancer date. The two modeling approaches used were general linear model controlling for patients' characteristics and calendar year, and general linear model with physician level indicator variables.
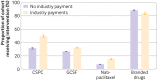
Results: Oncologists were in receipt of industry payments for 2962 of 9799 patients (30.2%) at risk for non-recommended denosumab (median $63), 76 747 of 271 485 patients (28.3%) at risk for GCSF (median $60); 18 491 of 86 394 patients (21.4%) at risk for nab-paclitaxel (median $89), and 4170 of 13 386 patients (31.2%) at risk for branded drugs (median $156). The unadjusted proportion of patients who received non-recommended denosumab was 31.4% for those whose oncologist had not received payment and 49.5% for those whose oncologist had (prevalence difference 18.0%); the corresponding values for GCSF were 26.6% v 32.1% (5.5%), for nab-paclitaxel were 7.3% v 15.1% (7.8%), and for branded drugs were 88.3% v 83.5% (-4.8%). Controlling for patients' characteristics and calendar year, payments from industry were associated with increased use of denosumab (17.5% (95% confidence interval 15.3% to 19.7%)), GCSF (5.8% (5.4% to 6.1%)), and nab-paclitaxel (7.6% (7.1% to 8.1%)), but lower use of branded drugs (-4.6% (-5.8% to -3.3%)). In physician level indicator models, payments from industry were associated with increased use of denosumab (7.4% (2.5% to 12.2%)) and nab-paclitaxel (1.7% (0.9% to 2.5%)), but not with GCSF (0.4% (-0.3% to 1.1%)) or branded drugs (1.2% (-6.0 to 8.5%)).
Conclusions: Within some clinical scenarios, industry payments to physicians are associated with non-recommended and low value drugs. These findings raise quality of care concerns about the financial relationships between physicians and industry.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/disclosure-of-interest/ and declare: support from the National Cancer Institute and Institute of Health Care management; no support from any organization for the submitted work for the submitted work. AMM declares stock ownership in DNA and Teladoc Health. NT declares employment and stock options at Delfi Diagnostics. PB declares consulting/advisory role at EQRx, leadership roles at Delfi Diagnostics and Oncology Analytics, travel expenses paid by Oncology Analytics, stock ownership at EQRx, Oncology Analytics, and Delfi Diagnostics, and research funding by Kaiser Permanente and Arnold Ventures. ANW declares consulting with Takeda and CorMedix.
Figures
Comment in
-
Use of denosumab in castration sensitive prostate cancer.BMJ. 2023 Dec 4;383:2827. doi: 10.1136/bmj.p2827. BMJ. 2023. PMID: 38049158 No abstract available.
Similar articles
-
Evaluating the Strength of the Association Between Industry Payments and Prescribing Practices in Oncology.Oncologist. 2019 May;24(5):632-639. doi: 10.1634/theoncologist.2018-0423. Epub 2019 Feb 6. Oncologist. 2019. PMID: 30728276 Free PMC article.
-
Physician Payments from Pharmaceutical Companies Related to Cancer Drugs.Oncologist. 2022 Oct 1;27(10):857-863. doi: 10.1093/oncolo/oyac160. Oncologist. 2022. PMID: 35946837 Free PMC article.
-
Increasing Financial Payments From Industry to Medical Oncologists in the United States, 2014-2017.J Natl Compr Canc Netw. 2021 Dec 29;20(13):1-9. doi: 10.6004/jnccn.2021.7024. J Natl Compr Canc Netw. 2021. PMID: 34965511 Free PMC article.
-
Are Financial Payments From the Pharmaceutical Industry Associated With Physician Prescribing? : A Systematic Review.Ann Intern Med. 2021 Mar;174(3):353-361. doi: 10.7326/M20-5665. Epub 2020 Nov 24. Ann Intern Med. 2021. PMID: 33226858 Free PMC article.
-
Industry Payments to Adult Reconstruction-Trained Orthopedic Surgeons: An Analysis of the Open Payments Database From 2014 to 2019.J Arthroplasty. 2021 Nov;36(11):3788-3795. doi: 10.1016/j.arth.2021.07.004. Epub 2021 Jul 15. J Arthroplasty. 2021. PMID: 34362596 Review.
Cited by
-
Trends in enforcement of National Comprehensive Cancer Network financial conflict of interest policy.JNCI Cancer Spectr. 2024 Nov 1;8(6):pkae120. doi: 10.1093/jncics/pkae120. JNCI Cancer Spectr. 2024. PMID: 39589914 Free PMC article.
-
Costs to Medicare of Nonrecommended Bone-Modifying Agent Use for Castration-Sensitive Prostate Cancer.JCO Oncol Pract. 2024 Mar;20(3):393-400. doi: 10.1200/OP.23.00602. Epub 2024 Jan 8. JCO Oncol Pract. 2024. PMID: 38190588 Free PMC article.
-
Use of Low-Value Cancer Treatments in Medicare Advantage Versus Traditional Medicare.J Clin Oncol. 2025 Jul 10;43(20):2245-2254. doi: 10.1200/JCO-24-01907. Epub 2025 May 31. J Clin Oncol. 2025. PMID: 40448575
-
Trends in financial payments from industry to US cancer centers, 2014-2021.JNCI Cancer Spectr. 2024 Apr 30;8(3):pkae015. doi: 10.1093/jncics/pkae015. JNCI Cancer Spectr. 2024. PMID: 38825338 Free PMC article.
-
Industry marketing payments to physicians and prescription patterns for sacubitril/valsartan in the USA.Heart. 2025 Jan 29;111(4):147-150. doi: 10.1136/heartjnl-2024-324453. Heart. 2025. PMID: 39542708 Free PMC article.
References
-
- The Facts About Open Payments Data: 2020 Totals. Centers for Medicare and Medicaid Services; 2022. Accessed April 15, 2022. https://openpaymentsdata.cms.gov/summary
-
- Institute of Medicine (US) Committee on Conflict of Interest in Medical Research, Education, and Practice. In: Lo B, Field MJ, eds. Conflict of Interest in Medical Research, Education, and Practice. National Academies Press, 2009, https://www.ncbi.nlm.nih.gov/books/NBK22942/, Accessed 24 Mar 2016. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous