Effects of PROtein enriched MEDiterranean Diet and EXercise on nutritional status and cognition in adults at risk of undernutrition and cognitive decline: the PROMED-EX Randomised Controlled Trial
- PMID: 37880167
- PMCID: PMC10603411
- DOI: 10.1136/bmjopen-2022-070689
Effects of PROtein enriched MEDiterranean Diet and EXercise on nutritional status and cognition in adults at risk of undernutrition and cognitive decline: the PROMED-EX Randomised Controlled Trial
Abstract
Introduction: Undernutrition leading to unplanned weight loss is common in older age and has been linked to increased dementia risk in later life. Weight loss can precede dementia by a decade or more, providing a unique opportunity for early intervention to correct undernutrition and potentially prevent or delay cognitive impairment. The combined effects of diet and exercise on undernutrition have not yet been evaluated. The objective of this trial is to determine the effect of a protein-enriched Mediterranean diet, with and without exercise, on nutritional status and cognitive performance in older adults at risk of undernutrition and cognitive decline.
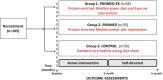
Methods: One hundred and five participants aged 60 years and over at risk of undernutrition and with subjective cognitive decline will be recruited to participate in a 6-month, single-blind, parallel-group randomised controlled trial. Participants will be block randomised into one of three groups: group 1-PROMED-EX (diet+exercise), group 2-PROMED (diet only) and group 3-standard care (control). The primary outcome is nutritional status measured using the Mini Nutritional Assessment. Secondary outcomes include cognitive function, nutritional intake, body composition, physical function and quality of life. Mechanistic pathways for potential diet and exercise-induced change in nutritional status and cognition will be explored by measuring inflammatory, metabolic, nutritional and metabolomic biomarkers.
Ethics and dissemination: The study is approved by the UK Office for Research Ethics Committee (ref: 21/NW/0215). Written informed consent will be obtained from participants prior to recruitment. Research results will be disseminated to the public via meetings and media and the scientific community through conference presentations and publication in academic journals.
Trial registration number: ClinicalTrials.gov Registry (NCT05166564).
Keywords: Dementia; NUTRITION & DIETETICS; Nutrition; PREVENTIVE MEDICINE; PUBLIC HEALTH.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
References
-
- Gabe S. Managing malnutrition to improve lives and save money. BAPEN, 2018.
-
- Guerin O, Soto ME, Brocker P, et al. Nutritional status assessment during Alzheimer’s disease: results after one year (the REAL French study group). J Nutr Health Aging 2005;9:81–4. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical